C6-alkylthio/amino substituted pyrimidine derivative and preparation method thereof
A technology of pyrimidine derivatives and alkylthio groups, which is applied in the field of C6-alkylthio/amino-substituted pyrimidine derivatives and their preparation, can solve the problems of lack of downstream operation space, complicated process of special oxidants, limited functional groups of products, etc. , to achieve the effects of cheap and easy availability of raw materials and catalysts, good functional group compatibility, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Preparation of Heterocyclic Compound 2a
[0030]
[0031] Wherein, SEt and EtS refer to thioethyl;
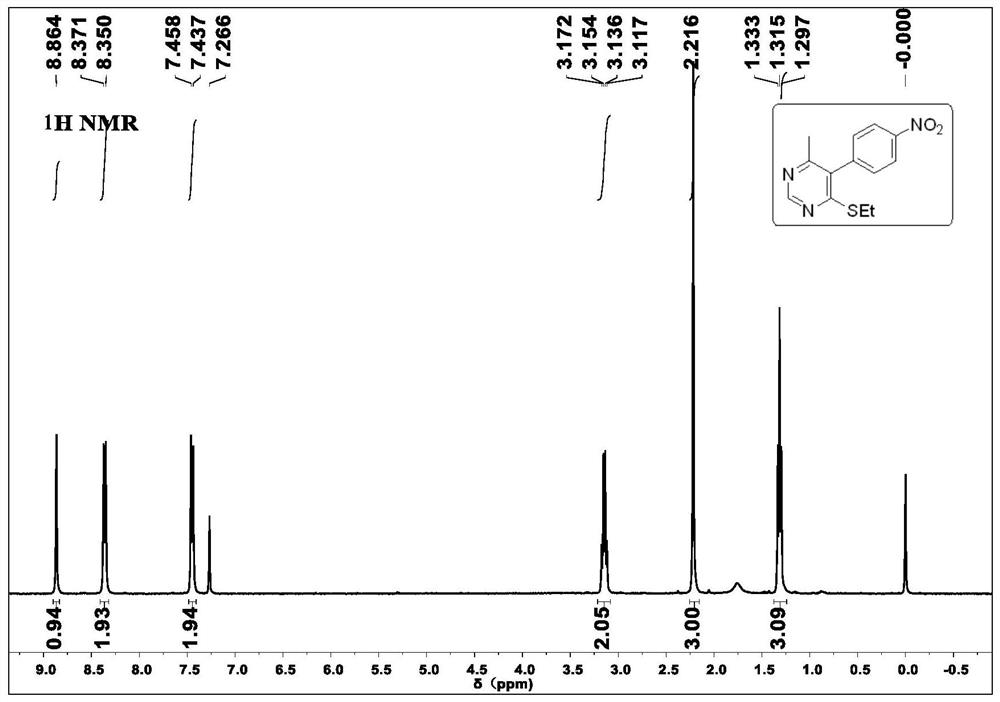
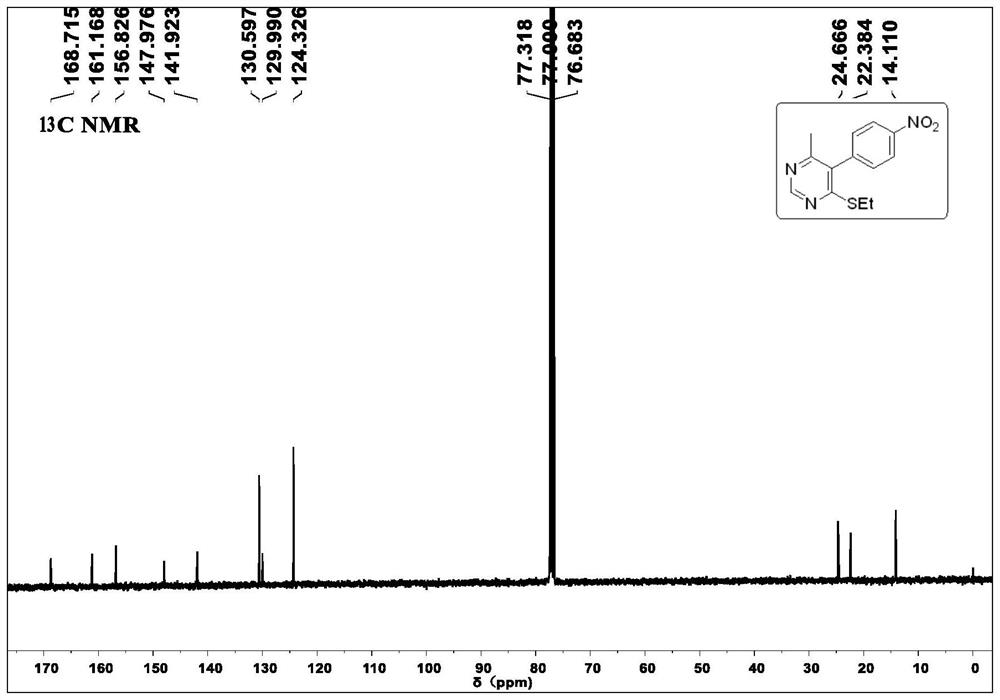
[0032] Under air conditions, 2-(bis(ethylthio)methylene)-3-oxo-N-phenylbutanamide 2a (309.1mg, 1mmol), ammonium acetate (462mg, 6mmol), copper chloride (13.4mg, 0.1mmol) mixed, added 10ml of methanol solvent, reacted at 90°C for 24h, filtered, extracted, dried, and evaporated under reduced pressure to remove the solvent, then chromatographed on a silica gel column to obtain a white solid 4-( Ethylthio)-6-methyl-N-phenylpyrimidine-5-carboxamide 2a (213.01 mg, yield 78%); relative to the general structural formula, the R of the product obtained in this embodiment 1 is hydrogen, R 2 is methyl, R 3 is an anilide group, R 4 For ethyl.
[0033] 1 H NMR (400MHz, CDCl 3 )δ1.37(t, J=7.2Hz, 3H), 2.50(s, 3H), 3.22(q, J=7.2Hz, 2H), 7.21(t, J=7.2Hz, 1H), 7.40(t, J=7.6Hz, 2H), 7.65(d, J=7.6Hz, 2H), 7.90(s, 1H), 8.79(s, 1H). 13 C NMR (100MHz, CDCl 3)δ14.2, 21.9, 24.4, 120....
Embodiment 2
[0035] Preparation of Heterocyclic Compound 2b
[0036]
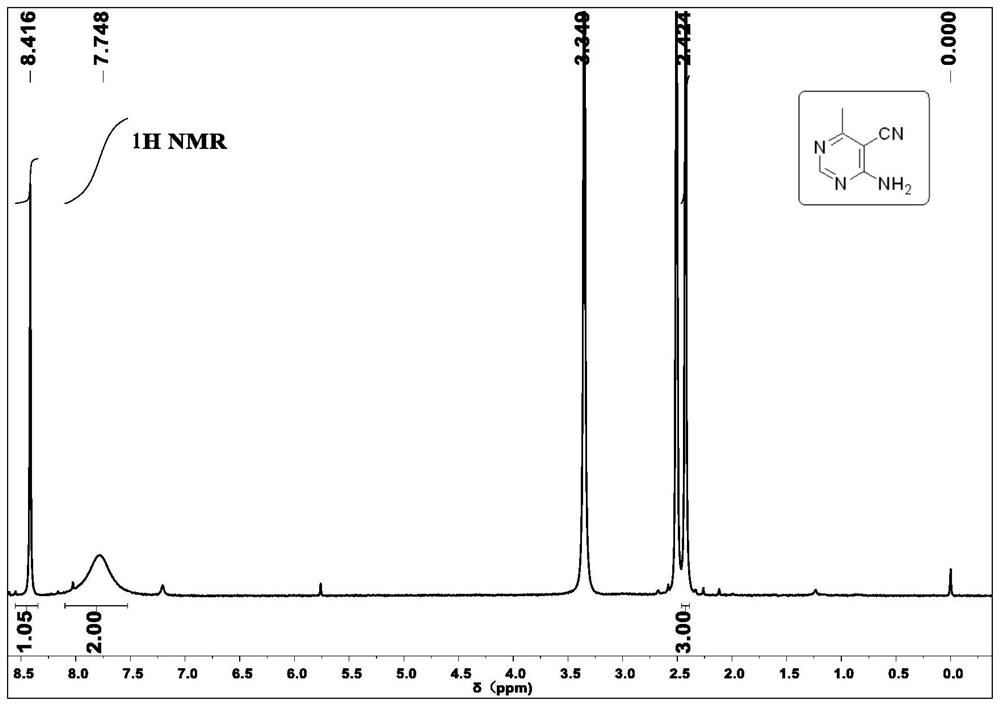
[0037] Under air conditions, 2-(bis(ethylthio)methylene)-3-oxobutyramide 2b (233.3 mg, 1 mmol), ammonium acetate (462 mg, 6 mmol), copper chloride (13.4 mg, 0.1 mmol) after mixing, add 10ml of methanol solvent, react at 90°C for 24h, filter with suction, extract, dry, and distill under reduced pressure to remove the solvent, then use silica gel column chromatography to obtain white solid 4-(ethylthio)- 6-methylpyrimidine-5-carboxamide 2b (155.7 mg, yield 79%);
[0038] 1 H NMR (400MHz, DMSO-d 6 )δ1.28(t, J=6.4Hz, 3H), 2.36(s, 3H), 3.16(q, J=7.2Hz, 2H), 7.84(s, 1H), 8.06(s, 1H), 8.82( s, 1H). 13 C NMR (100MHz, DMSO-d 6 )δ14.4, 21.5, 23.3, 129.0, 156.3, 160.0, 165.2, 166.5. HRMS (ESI-TOF) calcd for C 8 h 11 N 3 NaOS + ([M+Na] + )220.0515, found 220.0518.
Embodiment 3
[0040] Preparation of Heterocyclic Compound 2c
[0041]
[0042] Under air conditions, tert-butyl 2-(bis(ethylthio)methylene)-3-oxobutanoate 2c (290 mg, 1 mmol), ammonium acetate (462 mg, 6 mmol), copper chloride (13.4 mg , 0.1mmol) after mixing, add 10ml of methanol solvent, react at 90°C for 24h, filter, extract, dry, and distill under reduced pressure to remove the solvent, then use silica gel column chromatography to obtain yellow oil 4-(ethylsulfide Base)-tert-butyl 6-methylpyrimidine-5-carboxylate 2c (139.9 mg, yield 55%);
[0043] 1 H NMR (400MHz, CDCl 3 )δ1.37(t, J=7.4Hz, 3H), 1.63(s, 9H), 2.51(s, 3H), 3.19(q, J=7.2Hz, 2H), 8.79(s, 1H). 13 C NMR (100MHz, CDCl 3 )δ14.2, 22.7, 24.4, 28.1 (3C), 83.7, 125.5, 156.7, 162.4, 165.1, 168.2. HRMS (ESI-TOF) calcd for C 12 h 18 N 2 NaO 2 S + ([M+Na] + )277.0981, found 277.0972.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com