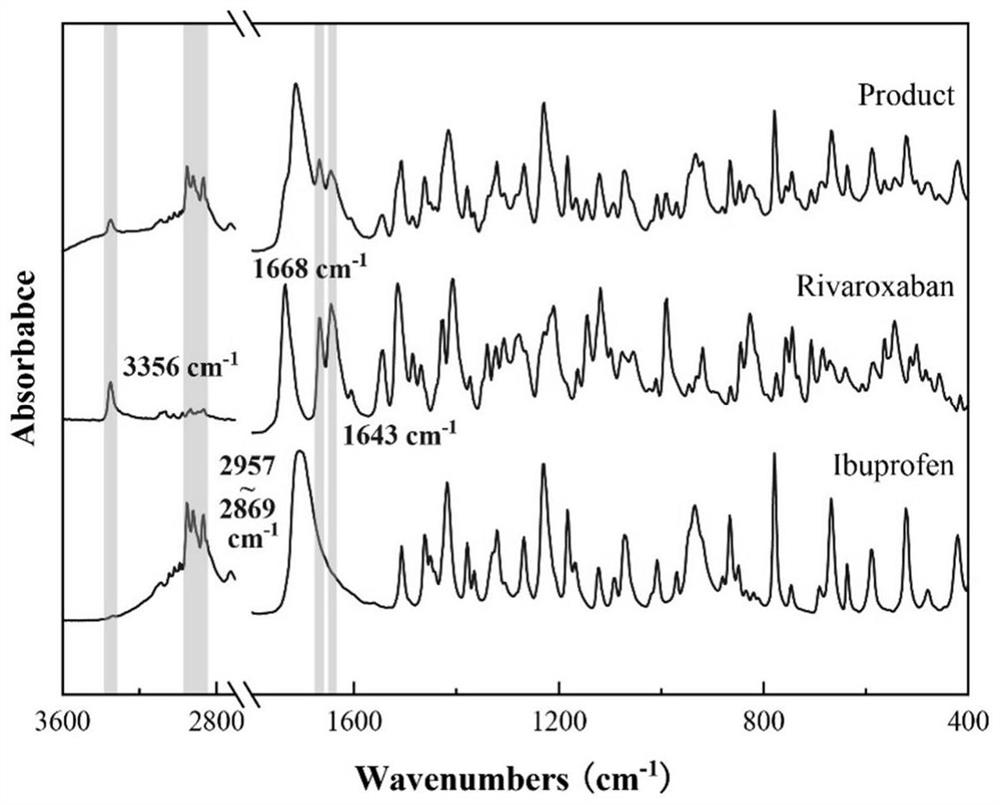
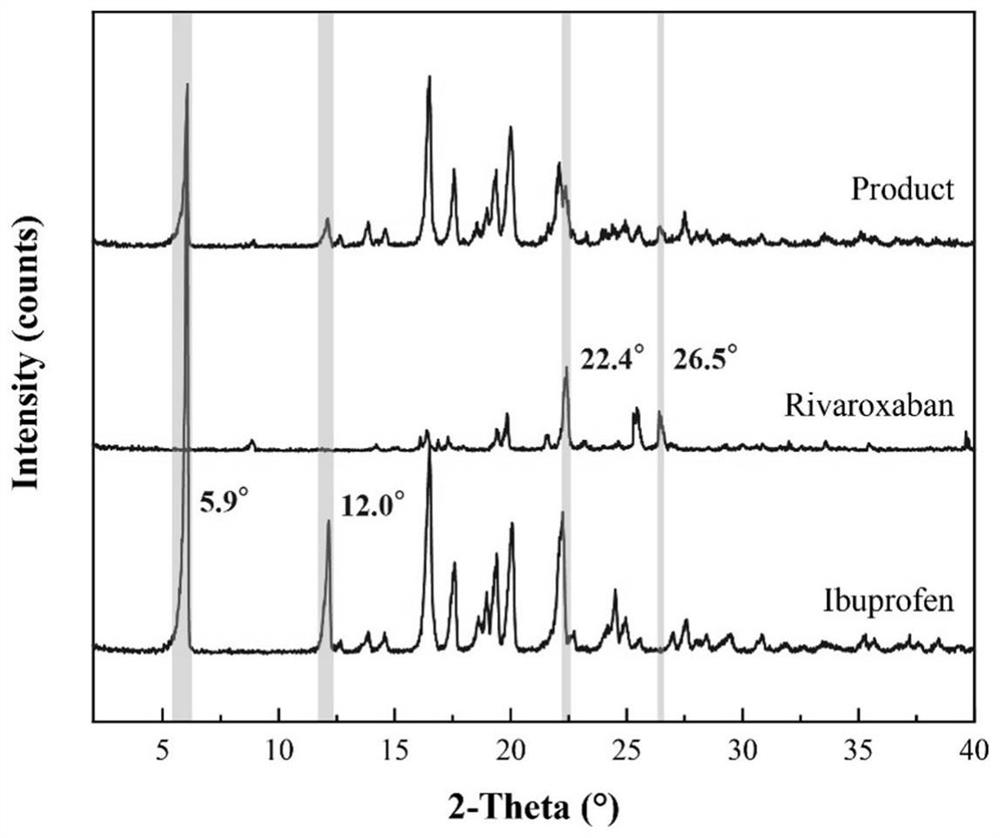
Ibuprofen-loaded rivaroxaban functional particles and preparation method thereof
A functional technology of rivaroxaban, applied in pharmaceutical formulations, organic active ingredients, non-central analgesics, etc., can solve the problems of complex equipment, high economic cost, poor mobility, etc., to reduce industrialization costs and economic investment The effect of low and excellent filling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] (1) At 80°C, prepare an aqueous solution with an ibuprofen concentration of 5.0 mg / mL, and stir until stratification occurs and the ibuprofen oil droplets are evenly distributed in the water;
[0036] (2) Keep the temperature and stirring rate constant, add rivaroxaban to the ibuprofen-water solution, wherein the concentration of rivaroxaban is 2.0mg / mL; continue stirring for 0.5h to make the rivaroxaban particles and cloth Profen oil droplets fully contact;
[0037] (3) Cool the solution to 1°C at a cooling rate of 80°C / 10min, and maintain a stirring power per unit volume of 2.093kW / m 3 To crystallization, add surfactant sodium stearate 0.02% (based on the quality of ibuprofen-rivaroxaban-water mixed solution) at this temperature. Stir continuously for 0.5h to make the crystals coalesce into compact particles;
[0038] (4) After vacuum filtration, washing with water, and drying at normal pressure at 25° C. for 12 hours, the ibuprofen-loaded rivaroxaban functional gra...
Embodiment 2
[0043] (1) At 85°C, prepare an aqueous solution with an ibuprofen concentration of 5.0 mg / mL, and stir until stratification occurs and the ibuprofen oil droplets are evenly distributed in the water;
[0044] (2) Keep the temperature and stirring rate constant, add rivaroxaban to the ibuprofen-water solution, wherein the concentration of rivaroxaban is 3.0mg / mL; continue stirring for 1.5h to make the rivaroxaban particles and cloth Profen oil droplets fully contact;
[0045] (3) Cool the solution to 5°C at a cooling rate of 60°C / 10min, and maintain a stirring power per unit volume of 0.732kW / m 3 To crystal out, add surfactant sodium hexametaphosphate 0.15% (based on the quality of ibuprofen-rivaroxaban-water mixed solution) at this temperature. Continue to stir for 2 hours to make the crystals coalesce into compact particles;
[0046] (4) After vacuum filtration, washing with water, and drying at 40° C. under normal pressure for 24 hours, the ibuprofen-loaded rivaroxaban func...
Embodiment 3
[0049] (1) At 90°C, prepare an aqueous solution with an ibuprofen concentration of 5.0 mg / mL, and stir until stratification occurs and the ibuprofen oil droplets are evenly distributed in the water;
[0050] (2) Keep the temperature and stirring rate constant, add rivaroxaban to the ibuprofen-water solution, wherein the concentration of rivaroxaban is 6.0mg / mL; continue stirring for 3h to make the rivaroxaban particles and ibuprofen The fen oil drops are in full contact;
[0051] (3) Cool the solution to 10°C at a cooling rate of 50°C / 10min, and maintain a stirring power per unit volume of 0.138kW / m 3 To crystal out, add surfactant sodium dodecylbenzene sulfonate 0.40% (based on the quality of ibuprofen-rivaroxaban-water mixed solution) at this temperature. Continue to stir for 3 hours to make the crystals coalesce into compact particles;
[0052] (4) After vacuum filtration, washing with water, and drying at normal pressure at 50° C. for 36 hours, the ibuprofen-loaded rivar...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| angle of repose | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com