Lentivirus purification process
A lentivirus and process technology, applied in the direction of virus, recovery/purification, virus/phage, etc., can solve the problems of increasing the risk of lentiviral particle degradation, intolerance to mechanical pressure and shear force, expensive purification medium, etc., to save effect of process operation time, prevention of degradation, and loss of recovery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
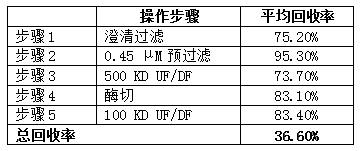
[0023] A lentivirus purification process, which comprises the following steps:
[0024] S1 Clarification and filtration of the cell culture of the adherent culture process is carried out with clarification and filtration membrane bag, clarification and filtration of the cell culture of the suspension cell culture process is carried out with the deep filter membrane bag, and the cell culture supernatant containing lentivirus is harvested, And remove cells, cell debris and large particle impurities, the turbidity of the supernatant is reduced to below 5 NTU;
[0025] S2 using a 300KD hollow fiber ultrafiltration system to concentrate the supernatant obtained in step S1 8 times;
[0026] S3 Use the first buffer solution to diafilter at least 10 volumes of the concentrated solution obtained in step S2 to remove impurities to the greatest extent. The first buffer solution includes 4-hydroxyethylpiperazineethanesulfonic acid, sucrose and MgCl 2 , whose concentrations were, 10 mM 4-...
Embodiment 2
[0031] A lentivirus purification process, which comprises the following steps:
[0032] S1 Clarification and filtration of the cell culture of the adherent culture process is carried out with clarification and filtration membrane bag, clarification and filtration of the cell culture of the suspension cell culture process is carried out with the deep filter membrane bag, and the cell culture supernatant containing lentivirus is harvested, And remove cells, cell debris and large particle impurities, the turbidity of the supernatant is reduced to below 5 NTU;
[0033] S2 adopts the hollow fiber ultrafiltration system of 750KD to concentrate the supernatant obtained in step S1 by 15 times;
[0034]S3 Use the first buffer solution to diafilter at least 10 volumes of the concentrated solution obtained in step S2 to remove impurities to the greatest extent. The first buffer solution includes 4-hydroxyethylpiperazineethanesulfonic acid, sucrose and MgCl 2 , whose concentrations were,...
Embodiment 3
[0039] A lentivirus purification process, which comprises the following steps:
[0040] S1 Clarification and filtration of the cell culture of the adherent culture process is carried out with clarification and filtration membrane bag, clarification and filtration of the cell culture of the suspension cell culture process is carried out with the deep filter membrane bag, and the cell culture supernatant containing lentivirus is harvested, And remove cells, cell debris and large particle impurities, the turbidity of the supernatant is reduced to below 5 NTU;
[0041] S2 using a 500KD hollow fiber ultrafiltration system to concentrate the supernatant obtained in step S1 by 15 times;
[0042] S3 Use the first buffer solution to diafilter at least 10 volumes of the concentrated solution obtained in step S2 to remove impurities to the greatest extent. The first buffer solution includes 4-hydroxyethylpiperazineethanesulfonic acid, sucrose and MgCl 2 , whose concentrations were, 50 m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com