Fusion enzyme and application of fusion enzyme in paper-based biosensor
A fusion enzyme, paper-based technology, applied in the field of biological enzyme genetic engineering and biosensing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0047] Furthermore, the present invention provides a specific preparation method, that is, transforming the coding gene into Pichia pastoris, and then carrying out the steps of expression and purification.
[0048] The vector used in the present invention is not particularly limited as long as it can replicate in a host cell, and any known vector in the art can be used. Examples of conventional vectors may include natural or recombinant plasmids, cosmids, viruses and phages. For example, pWE15, M13, MBL3, MBL4, IXII, ASHII, APII, t10, t11, Charon 4A, and Charon 21A can be used as phage vectors or cosmid vectors. As the plasmid vector, pBR type, pUC type, pBluescriptII type, pGEM type, pTZ type, pCL type and pET type can be used. The vector usable in the present invention is not particularly limited, and any known expression vector can be used. Preferably, pDZ, pPIC9k, pACYC184, pCL, pECCG117, pUC57, pBR322, pMW118 or pCC1BAC vectors can be used.
[0049] The second aspect o...
Embodiment 1
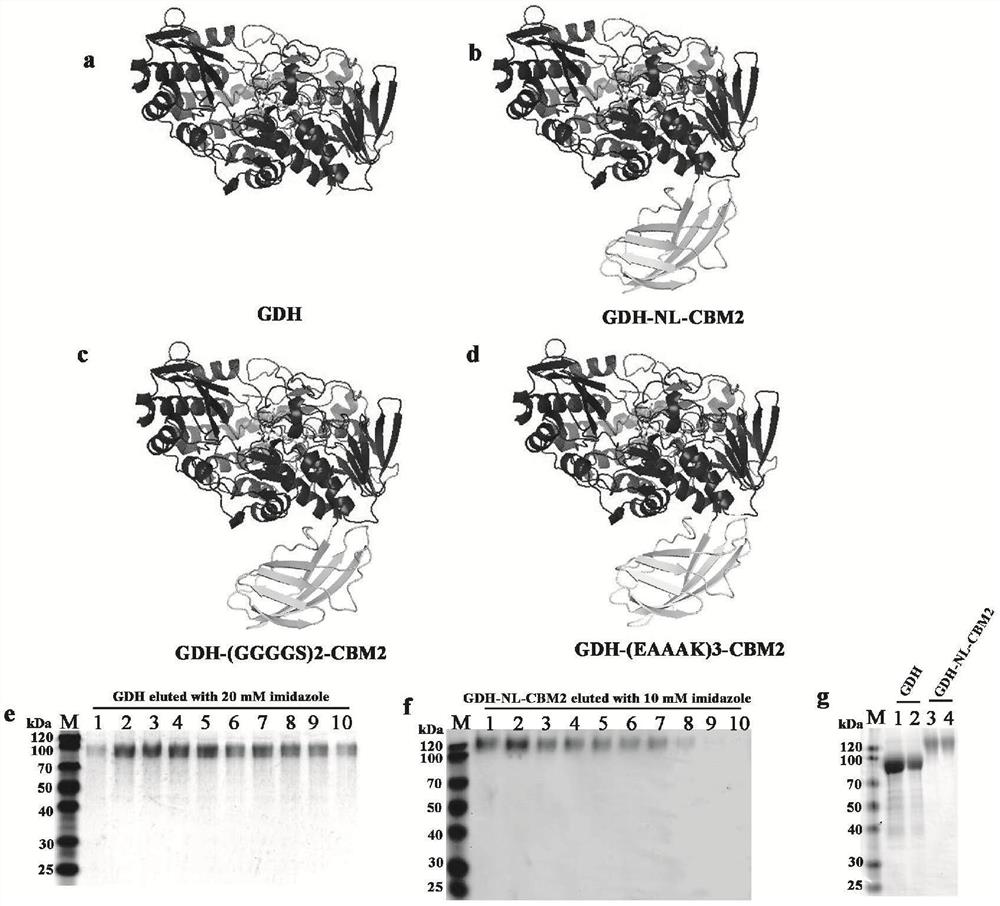
[0057] Embodiment 1, fusion expression of CBM and GDH
[0058] Select CBMs sequences with binding specificity to cellulose from CBM1, CBM2, CBM3, CBM5, and CBM10 families, and use structural bioinformatics analysis tools (SWISS-MODEL, ClustalX, VMD, and PyMOl software) to analyze the amino acid frequency and function of different CBMs. Statistical analysis was performed on information such as the framework sequence profile, and CBM2 in Thermobifida fusca was screened out for fusion enzyme construction.
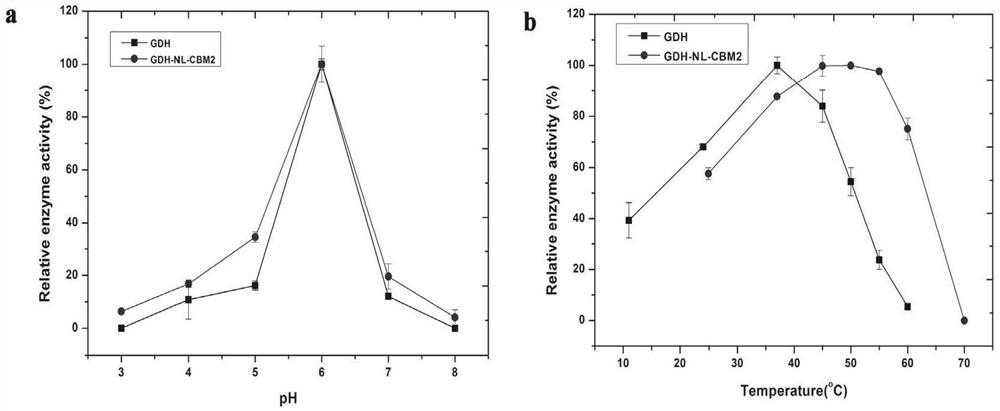
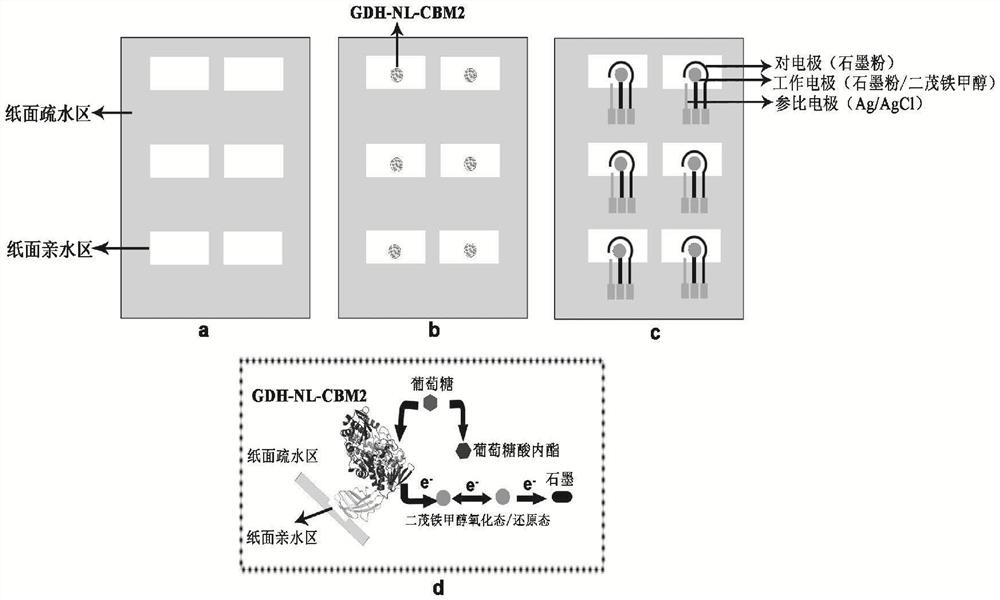
[0059] Based on the analysis of the structural characteristics of CBM2 and GDH, the fusion enzyme molecular linker sequences ((GGGGS)2, (EAAAK)3) were selected from the LinkerDB database, and the endo-β-xylanase (EM_PRO:Z81013. 1) The natural linker sequence connecting the catalytic domain and the CBM2 domain; respectively used as a connecting peptide for the construction of GDH fusion enzyme, the natural linker sequence is "LGGDSSGGGPGEPGGPGGPGEPGGPGGPGEPGGPGDGT", the predict...
Embodiment 2
[0061] Using the pPIC9k-GDH plasmid connected with the codon-optimized Aspergillus niger An76 GDH gene obtained in the previous study as a template, primers were designed to obtain the gene encoding GDH, and the plasmid pUC57-linker-CBM2 DNA was used as a template to design primers to obtain the linker and CBM2 gene. Three kinds of GDH fusion enzyme genes with different connecting peptides were obtained according to the method of Nanjing Nuoweizan homologous recombination kit. The three kinds of GDH fusion enzyme genes were respectively expressed with pPIC9K plasmid and Pichia pastoris GS115 as the expression host to realize GDH fusion enzyme Express.
[0062] (1) GDH fusion enzyme gene cloning:
[0063] Using the principle of homologous recombination and the sequence near the Pichia pastoris GS115 cloning site, six pairs of primers were designed according to the codon-optimized GDH gene sequence and pUC57-linker-CBM2 gene sequence:
[0064]
[0065]
[0066] Using the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com