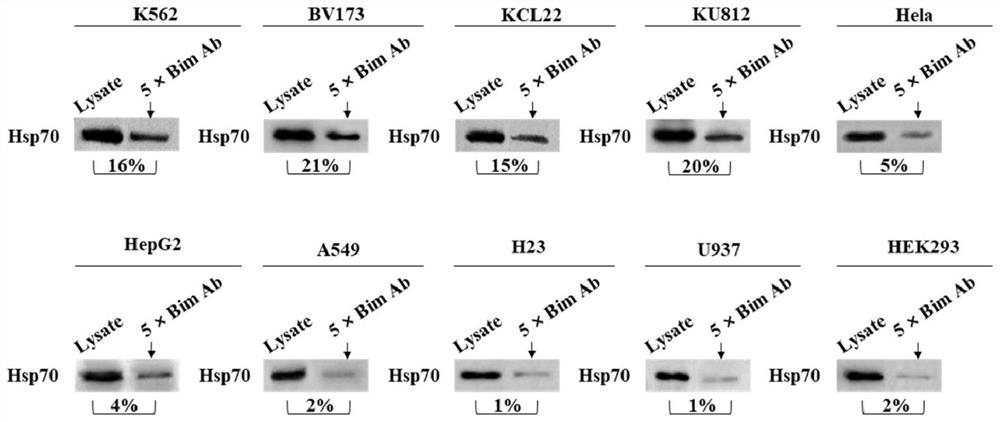
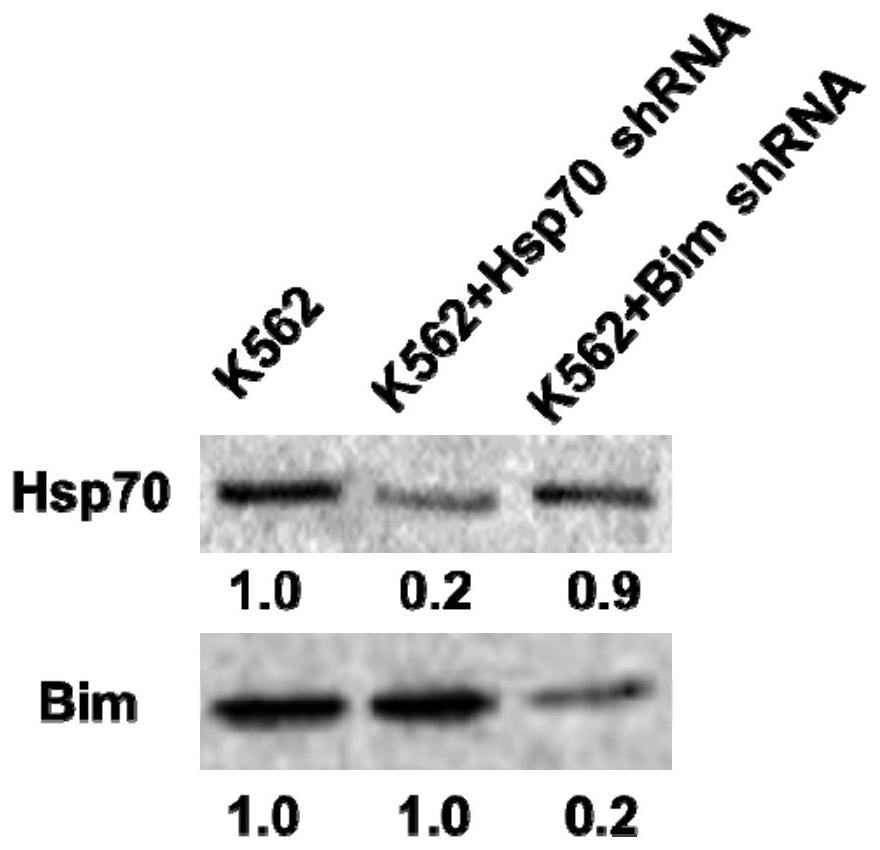
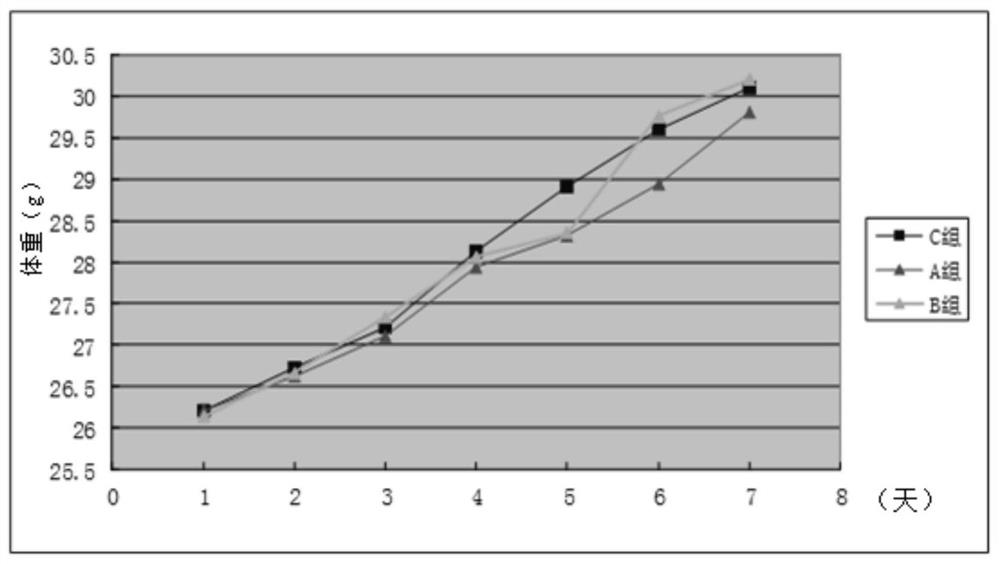
2-cyano phenalenone compound and application thereof in treatment of leukemia
A technology of cyanophenadenone and compounds, applied in the field of 2-cyanophenadenone compounds and their application in the treatment of leukemia, capable of solving problems such as interference and killing tumor cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1: Preparation of 6-cyclohexylsulfanyl-2,3-dicyano-phenadenone (compound 1)
[0051]
[0052] (1) Synthesis of Intermediate A1
[0053] Take 920mg (4.0mmol) of 2,3-dicyanophenadenone and 1162mg (10mmol) of cyclohexanethiol respectively, add 100mL of acetonitrile, react at room temperature for 6h, and remove the solvent by rotary evaporation. The obtained solid was separated by silica gel column chromatography, and the developing solvent was CH 2 Cl 2 :CH 3 OH=100:1 (v / v), the intermediate A1 was obtained as a red solid with a yield of 25%.
[0054] (2) Synthesis of compound 1
[0055] 172 mg (0.5 mmol) of intermediate A1 and 3-amino-1-propanol (10 mmol) were dissolved in 30 mL of acetonitrile, reacted at room temperature for 3 h, and the solvent was removed by rotary evaporation. The obtained solid was separated by silica gel column chromatography, and the developing solvent was CH 2 Cl 2 :CH 3 OH=100:1 (v / v), the compound 1 was obtained as a yellow s...
Embodiment 2
[0057] Embodiment 2: the preparation of compound 2-27
[0058] Similar to Example 1, compound 2-27 was synthesized. Wherein the yield and characterization data of compound 2-27 are as follows:
[0059] Compound 2: Yield 14%. 1 H-NMR (500MHz,D 6 -DMSO): δ8.67(d, J=8.4Hz, 1H), 8.46(t, J=8.4Hz, 1H), 8.02(t, J=8.4Hz, 1H), 7.75(t, J=8.4Hz ,1H),7.68(t,J=8.4Hz,1H),3.73(t,J=6.4Hz,2H),3.62(s,3H),2.61(t,J=6.4Hz,2H),2.84(m ,J=4.4Hz,1H),1.98(d,J=4.4Hz,2H),1.87(d,J=4.4Hz,2H),1.73(d,J=4.4Hz,2H),1.65(t,J =4.4Hz, 2H), 1.43(d, J=4.4Hz, 2H).ESI-MS: (C 24 h 24 N 2o 3 S[M+H] + ): 421.16.
[0060] Compound 3: The yield is 22%. 1 H-NMR (500MHz,D 6 -DMSO): δ8.67(d, J=8.4Hz, 1H), 8.46(t, J=8.4Hz, 1H), 8.02(t, J=8.4Hz, 1H), 7.75(t, J=8.4Hz ,1H),7.68(t,J=8.4Hz,1H),3.73(t,J=6.4Hz,2H),3.55(t,J=4.2Hz,4H),2.61(t,J=6.4Hz,2H ), 2.84(m, J=4.4Hz, 1H), 2.47(t, J=4.2Hz, 4H), 1.98(d, J=4.4Hz, 2H), 1.87(d, J=4.4Hz, 2H), 1.73(d, J=4.4Hz, 2H), 1.65(t, J=4.4Hz, 2H), 1.43(d, J=4.4Hz, 2H); 13 C-NMR (125...
Embodiment 3
[0085] Embodiment 3: the preparation of compound 28
[0086]
[0087] 42 mg (0.1 mmol) of compound 2 was dissolved in 20 mL of methanol, and 2 mL of 10 mM NaOH aqueous solution was added dropwise at 0°C with stirring, and reacted at room temperature for 12 h. 50 mL of deionized water was added to the reaction solution, and the pH was adjusted to 2 with 1M hydrochloric acid to generate a yellow solid. After suction filtration, the resulting solid was rinsed three times with methanol (5 mL) at 0° C. to obtain compound 28 as a yellow solid with a yield of 91%.
[0088] Compound 28 1 H NMR measurement result and mass spectrometry result are as follows: 1 H-NMR (500MHz,D 6 -DMSO): δ8.64(d, J=8.0Hz, 1H), 8.46(t, J=8.0Hz, 1H), 8.00(t, J=8.0Hz, 1H), 7.72(t, J=8.0Hz ,1H),7.64(t,J=8.0Hz,1H),6.46(s,1H),4.37(t,J=6.4Hz,2H),2.85(m,J=4.0Hz,1H),2.76(t ,J=6.4Hz,2H),2.01(d,J=4.0Hz,2H),1.87(d,J=4.0Hz,2H),1.74(m,J=4.0Hz,2H),1.68(d,J =4.0Hz, 2H), 1.44(d, J=4.0Hz, 2H); ESI-MS (C 23 h 23 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com