New impurity and preparation method thereof
A technology of impurities and pyrrolidinyl, which is applied in the field of new impurities and its preparation, can solve the problems affecting the quality level of finished products, and achieve the effects of short synthesis route, high product purity and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1 Preparation of 6-bromo-3-methyl-10-(2-(1-pyrrolidinyl)ethyl)pyrido[1,2-α]indole (recovery crystallization method from mother liquor)
[0060]Concentrate about 4.5L (E)-2-bromo-6-(3-(1-pyrrolidinyl)-1-p-tolyl-1-propenyl)pyridine (Formula III) mother liquor at 50-60°C under reduced pressure to about 200-300ml, then add about 5L of drinking water and 1L of ethyl acetate to the concentrated residue, add ammonia water under stirring to adjust the pH to 8-10, after the adjustment is completed, let it stand for stratification, separate the organic layer and then use 500ml The aqueous layer was extracted once with ethyl acetate; the combined organic layers were washed three times with saturated aqueous sodium chloride solution. Add 20g of activated carbon and 180g of anhydrous sodium sulfate to the organic layer, dry for 30 minutes, filter with suction, concentrate the mother liquor to dryness under reduced pressure, add about 600g of n-hexane, stir and dissolve at 60...
Embodiment 2
[0062] HPLC, IR, m / z, Proton spectrum and carbon spectrum identification, its result is as follows:
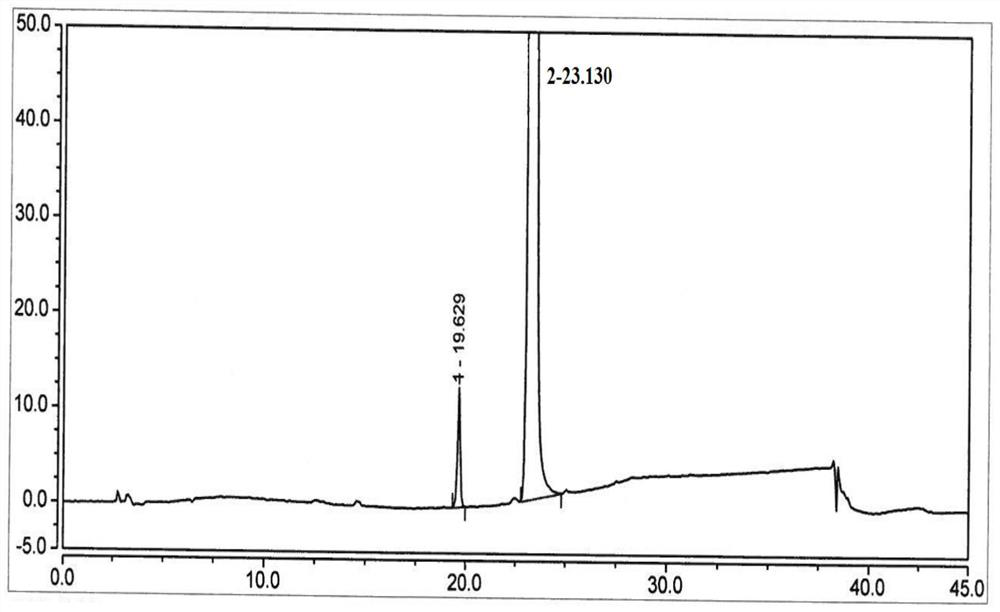
[0063] HPLC: 99.0% (with figure 1 ).
[0064] The integral result of HPLC is shown in the following table:
[0065]
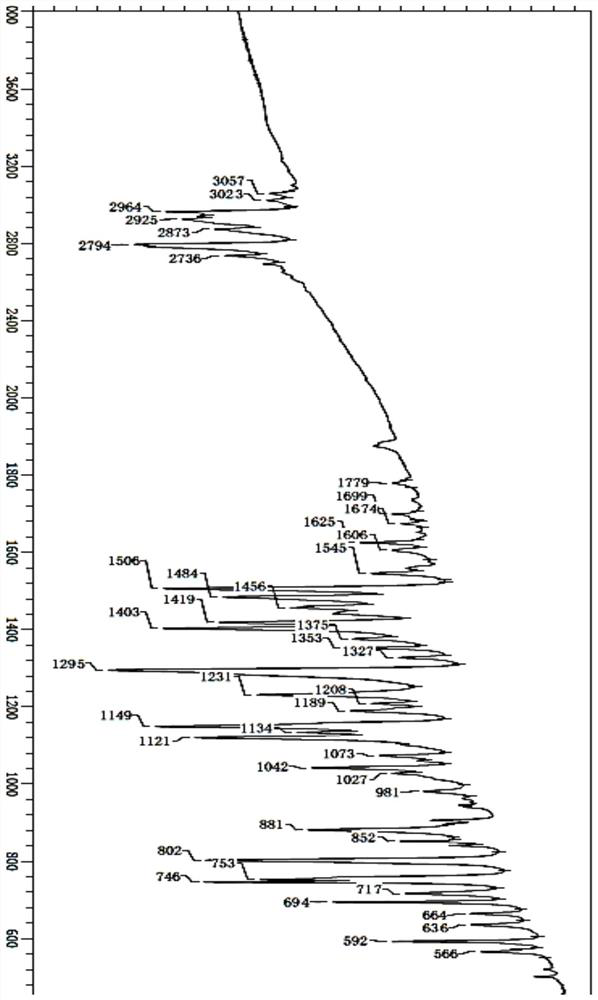
[0066] IR: 1699cm-1, 1674cm-1, 1625cm-1, 1606cm-1, 1484cm-1, 2964cm-1, 2873cm-1, 1375cm-1 (with figure 2 ).
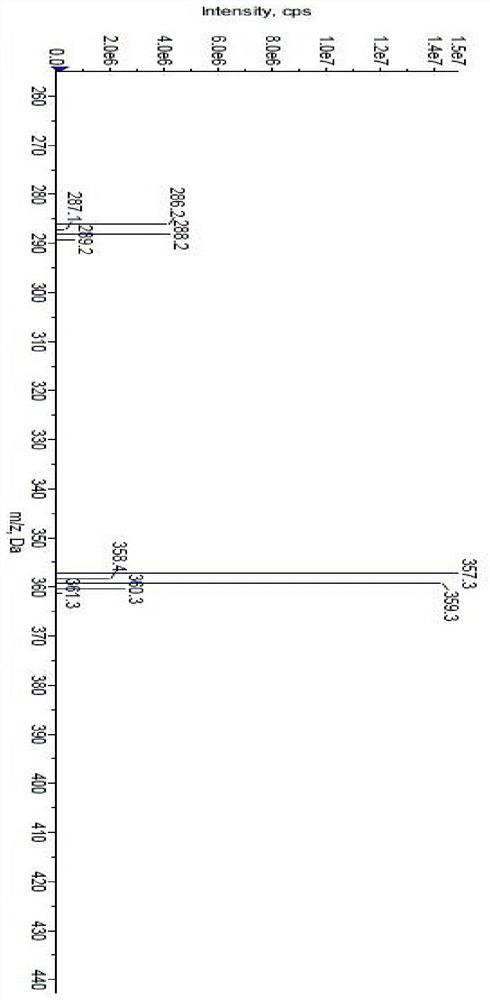
[0067] m / z: 357.3, 359.3[M+H]+ (with image 3 ).
[0068] 1HNMR (600MHz, CD3OD): 8.816(s,1H); 7.684-7.698(d,1H); 7.478-7.494(dd,1H); 7.245-7.259; 6.707-6.720; 2H); 2.729-2.756(m, 2H); 2.674-2.683(m, 4H); 2.541(s, 3H); 1.846-1.868(m, 4H) (attached Figure 4 ).
[0069] 13CNMR (600MHz, CD3OD): 136.514; 132.314; 130.145; 128.221; 125.347; 120.657; 118.396; 116.932; Figure 5 ).
Embodiment 3
[0070] Example 3 Preparation of bromo-3-methyl-10-(2-(1-pyrrolidinyl)ethyl)pyrido[1,2-α]indole (directed synthesis method)
[0071] Add 20g of water to a 500ml three-necked flask, slowly add 184g of sulfuric acid, and control the temperature below 90°C; add 50g of (E)-2-bromo-6-(3-(1-pyrrolidinyl)-1-p-tolyl- 1-propenyl)pyridine (Formula III), react at 108-120° C. for 4 hours at a temperature of 108 to 120° C., take a sample and carry out central control until the reaction meets the requirements. Cool down to 15-20°C, add ammonia water dropwise to adjust the pH value to 8-10, extract with 200ml×3 ethyl acetate, combine the organic layers, wash with 200ml×3 water, and concentrate to dryness. To obtain an oily substance, add 10ml of ethyl acetate and 50ml of n-hexane, heat up and reflux to dissolve, add dropwise 150ml of n-hexane, slowly cool down to crystallize, crystallize at 0-10°C for 1-2 hours, filter, wash with 20ml of refrigerated n-hexane, pump Dry; send solid samples fo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com