Method for detecting peramivir trihydrate by reversed-phase high performance liquid chromatography
A technology of peramivir trihydrate and reversed-phase high-performance liquid phase, which can be applied to measurement devices, instruments, scientific instruments, etc., can solve the difficulty of mobile phase pH screening, affect the quality of peramivir, drug safety, and quality differences bigger problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
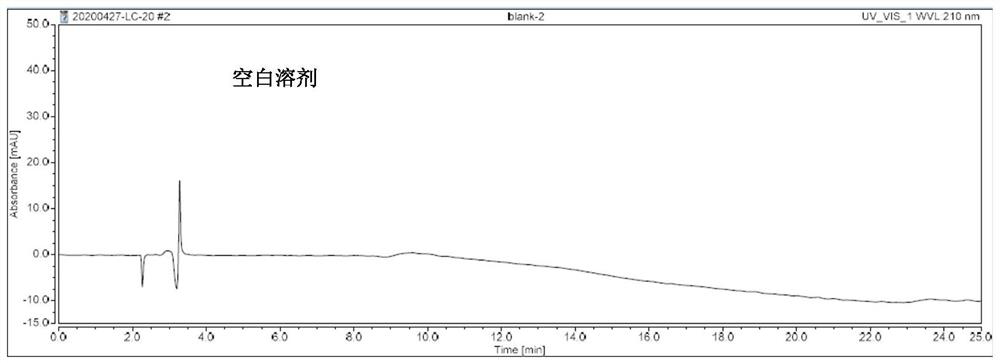
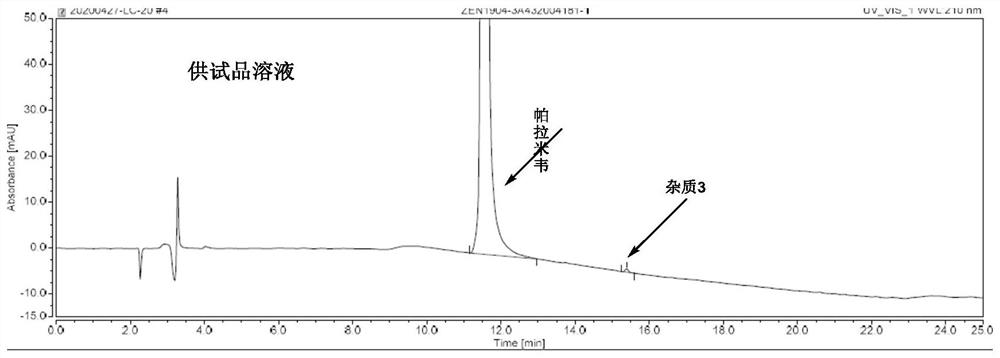
[0033] Example 1: Specificity test
[0034] Solvent: mobile phase A-mobile phase B=92:8 (V:V)
[0035] Impurity 1 mother liquor: Weigh 6 mg of Impurity 1 reference substance, accurately weigh it, put it in a 10mL volumetric flask, add solvent to dissolve and dilute to the mark, shake well, and prepare a solution with a concentration of Impurity 1 reference substance of 0.6 mg / mL.
[0036] Impurity 2 mother liquor: Weigh 6 mg of Impurity 2 reference substance, accurately weigh it, put it in a 10 mL volumetric flask, add solvent to dissolve and dilute to the mark, shake well, and prepare a solution with a concentration of Impurity 2 reference substance of 0.6 mg / mL.
[0037] Impurity 3 mother liquor: Weigh 6 mg of Impurity 3 reference substance, accurately weigh it, put it in a 10 mL volumetric flask, dissolve it with a solvent, dilute to the mark, shake well, and prepare a solution with a concentration of Impurity 3 reference substance of 0.6 mg / mL.
[0038] Peramivir mother s...
Embodiment 2
[0051] Example 2: Sensitivity test
[0052] Impurity Quantitative Limit Solution: Take Impurity 1, Impurity 2, and Impurity 3 under the specificity item, put them in different 50mL volumetric flasks, add solvent to dilute to the mark, shake well, and then precisely measure 3mL separately and place them in the same 100mL volumetric flask , dilute to the mark with solvent, and shake well. Then precisely measure 3mL of this solution and put it in a 5mL volumetric flask, add solvent and dilute to the mark.
[0053] Impurity detection limit solution: Precisely measure 3mL of the limit of quantification solution, put it in a 10mL volumetric flask, add solvent to dilute to the mark.
[0054] Peramivir limit of quantitation solution: Weigh 16 mg of peramivir reference substance, accurately weigh it, put it in a 20 mL volumetric flask, add solvent to dissolve and dilute to the mark, and shake well. Precisely measure 1mL, put it in a 50mL measuring bottle, add solvent to dilute to the...
Embodiment 3
[0060] Example 3: Linearity test
[0061] Dilute peramivir and its impurity reference substance solution with diluent to prepare a series of concentration reference solutions, inject into liquid chromatograph, and record the chromatogram. The results are shown in Tables 3-6.
[0062] Table 7 Impurity 1 standard curve
[0063]
[0064]
[0065] Table 8 Impurity 2 Linearity
[0066]
[0067]
[0068] Table 9 Impurity 3 Linearity
[0069]
[0070] Table 10 Peramivir linearity
[0071]
[0072]
[0073] It can be seen from Tables 7-10 that within the range of 0.2-3 μg / ml, the detection method of the present invention has a good linear relationship.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com