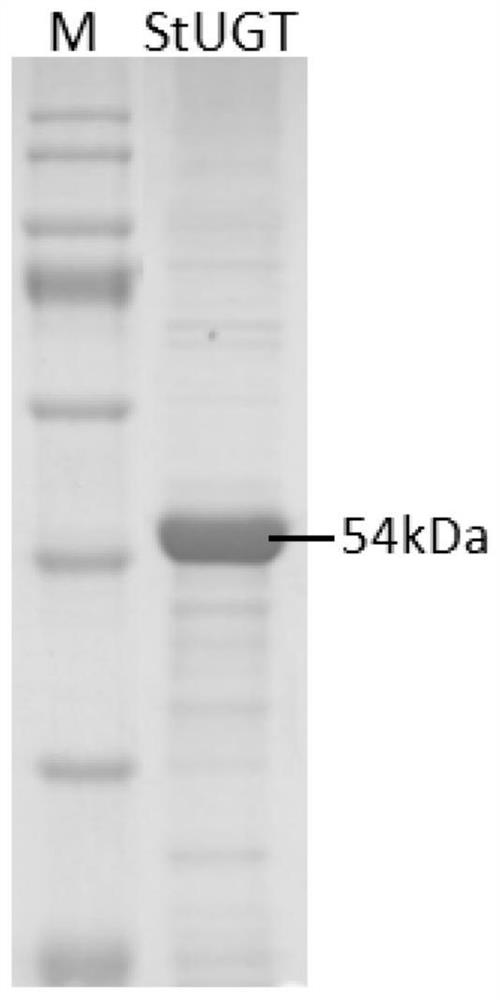
Glycosyltransferase StUGT capable of catalyzing rebaudioside A to generate rebaudioside D
A technology of glycosyltransferase and glycosylation reaction, applied in the field of bioengineering, can solve problems such as poor catalytic efficiency, and achieve the effect of high catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Acquisition of Glycosyltransferase StUGT Gene and Construction of Recombinant Strain
[0068] The potato (Solanum tuberosum) glycosyltransferase amino acid sequence (GenBank: XP_006367681.1; SEQ ID NO: 2) and nucleic acid sequence (GenBank: XM_006367619.1; SEQ ID NO: 1) were downloaded from GenBank. The corresponding sequence is shown in Table 1, and the nucleic acid sequence is codon-optimized by GenScript Company, and the optimized synthetic gene is connected to the vector pET28a(+) through the restriction site Nco1 and Xho1 to obtain the plasmid pET28a(+)-StUGT, For plasmid map see figure 1 .
[0069] The obtained plasmid pET28a-StUGT was transformed into E.coli BL21 competent cells, using LB (1% peptone, 0.5% yeast powder, 1% NaCl, 1.6% agar powder) solid plate containing 50 μg / ml kanamycin. After screening, the screened monoclonal transformants were identified by colony PCR to obtain a recombinant strain E.coli BL21 (pET28a-StUGT).
[0070] The above recombinant...
Embodiment 2
[0076] Induced expression of recombinant strain and preparation of crude enzyme solution
[0077] Taking the recombinant strain BL21(DE3)(pET28a-StUGT) as an example, the expression mode of the glycosyltransferase gene StUGT in E. coli is illustrated.
[0078] Strain BL21(DE3) (pET28a-StUGT) was grown to OD in LB liquid medium (1% peptone, 0.5% yeast powder, 0.5% NaCl) containing 50 μg / ml kanamycin at 37°C, 220 rpm 600 In 0.4-1.2, isopropyl-β-D-thiogalactoside (IPTG) was added to make the final concentration of the bacterial solution 0.1-1 mM, and expression was induced at 18°C-30°C for 5-17h.
[0079] The culture of induced expression was centrifuged (12000rpm, 4°C, 10min), the supernatant was discarded, and the cell pellet was collected. The collected cells were washed once with 10 mM PBS (pH 7.2) to remove the remaining medium on the cells; the cells were resuspended with 10 mM PBS (pH 7.2) at a ratio of 1 / 20 of the original cell volume, The cells were disrupted by ultras...
Embodiment 3
[0082] StUGT catalyzes the glycosylation of rebaudioside A to recitidine D
[0083] Using Rebaudioside A as a substrate, StUGT catalyzes the reaction system to generate Rebaudioside D as follows: Add RebA with a final concentration of 1-100 g / L, and uridine diphosphate glucose (UDPG) with a final concentration of 1-3 mM. ) with a final concentration of 3 mM Mg 2+ (magnesium chloride), add the crude enzyme liquid of the recombinant bacteria StUGT obtained in Example 2.
[0084] After the glycosylation reaction system is prepared, the reaction is allowed to stand at 18-45°C for 6-48h. After the reaction was completed, 500 μL of 60% (v / v) acetonitrile was added, and after shaking and mixing, centrifuged at 12,000 rpm for 10 min at room temperature, and the supernatant was passed through a 0.2 μm organic membrane and then analyzed by HPLC. HPLC used a Luna C18 reverse-phase bonded silica gel separation column (4.6mm×250mm, 5μm), the mobile phase was acetonitrile: sodium phosphat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com