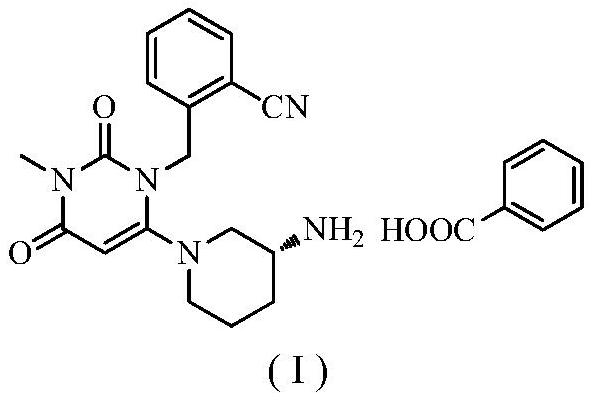
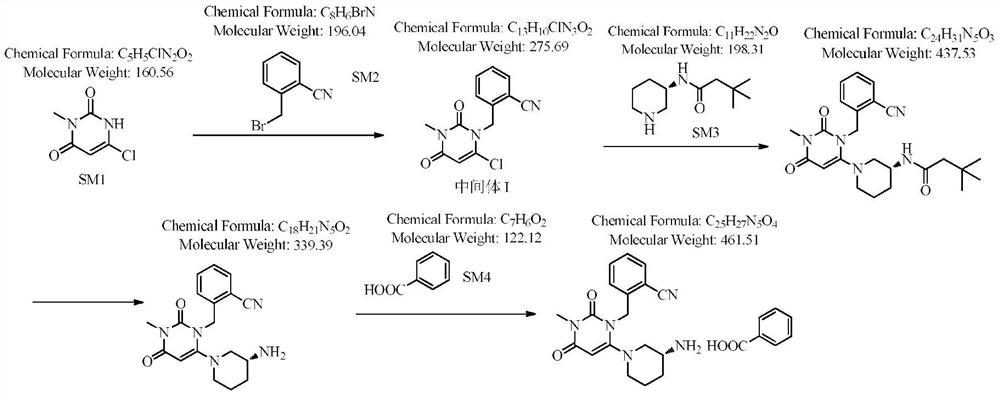
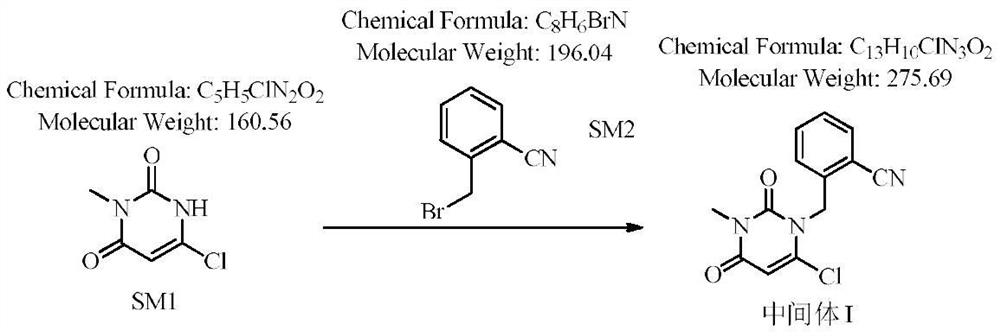
Novel preparation process of alogliptin benzoate
A technology of benzoic acid and methyl, which is applied in the field of drug synthesis and can solve problems such as waste water defects, three waste treatment costs, labor costs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Step 1: Preparation of 2-[(6-chloro-3,4-dihydro-3-methyl-2,4-dioxo-1(2H)-pyrimidinyl)methyl]benzonitrile
[0040] 100 g of 3-methyl-6-chlorouracil, 184.1 g of 2-cyanobenzyl bromide (1.1 equivalents), 800 ml of toluene, and 117.6 g of sodium carbonate (1.3 equivalents) were added to the reaction flask. The reaction was stirred at 60-70° C., detected by TLC, and the reaction was complete in 3 hours, which was directly used in the next reaction.
[0041] Step 2: Preparation of Alogliptin
[0042] Add 129.7 g of (R)-3-aminopiperidine dihydrochloride (1.05 equivalents) to the reaction flask of the first step above, stir and react at 60-70° C., detect by TLC, and complete the reaction in 7 hours. After filtering, the solvent of the filtrate was evaporated to dryness to obtain Boc-alogliptin.
[0043] Step 3: Preparation of Alogliptin
[0044] Add the above-mentioned Boc-trexagliptin to 400 ml of trifluoroacetic acid, stir and react at 30-40° C., TLC detects that the reacti...
Embodiment 2
[0048] Different from Example 1: the first and second step reaction bases are potassium carbonate (1.1 equivalents), and an off-white solid alogliptin benzoate is obtained. The total yield of the four-step reaction is 68.8%, and the HPLC purity is 99.67%.
Embodiment 3
[0050] Different from Example 1: the first and second reaction bases are sodium carbonate (1.5 equivalents), the total yield of the four-step reaction is 67.1%, and the HPLC purity is 99.69%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com