Inactivated whole-virus influenza vaccine and method for preparing same
An influenza vaccine, whole particle technology, applied in the field of inactivated whole particle influenza vaccine, can solve the problem of low antibody induction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] The preparation method of the inactivated whole particle influenza vaccine of the present invention utilizes the chicken embryo method and includes the process of performing hypotonic treatment.
[0036] The "chicken embryo method" refers to the method of inoculating the virus strain into hatching eggs for cultivation, clarifying, concentrating, purifying and inactivating the virus suspension to obtain a virus liquid containing virus particles.
[0037] Here, in the cultivation, the influenza virus strain is inoculated into hatching eggs, and it is performed at 30-37° C. for about 1 to 7 days, preferably at 33-35° C. for about 2 days. After the culture, the virus suspension (infected allantoic fluid) was collected and clarified by centrifugation or filtration. Next, it was concentrated by ultrafiltration. Virus purification can be performed by methods such as ultracentrifugation such as sucrose density gradient centrifugation, liquid chromatography, and the like.
[0...
Embodiment
[0053] Hereinafter, although an Example demonstrates this invention concretely, this invention is not limited by these at all.
reference example 1
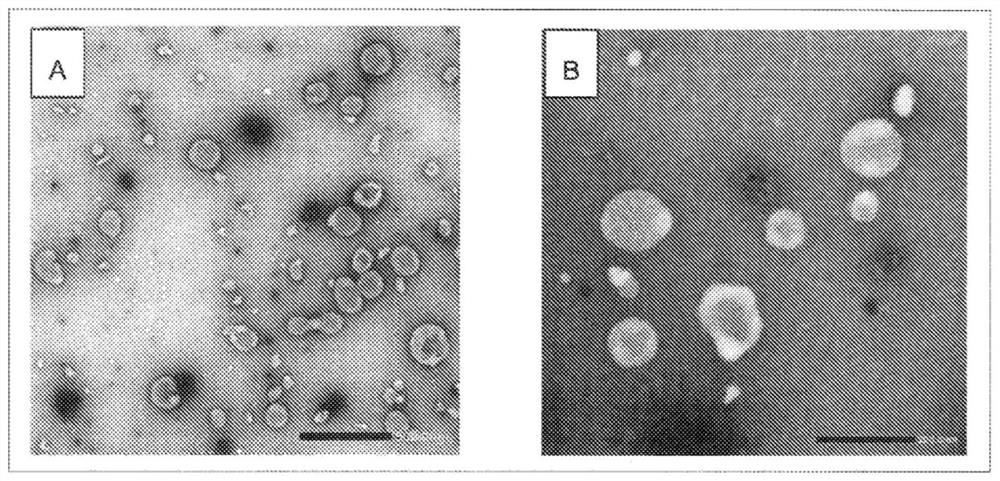
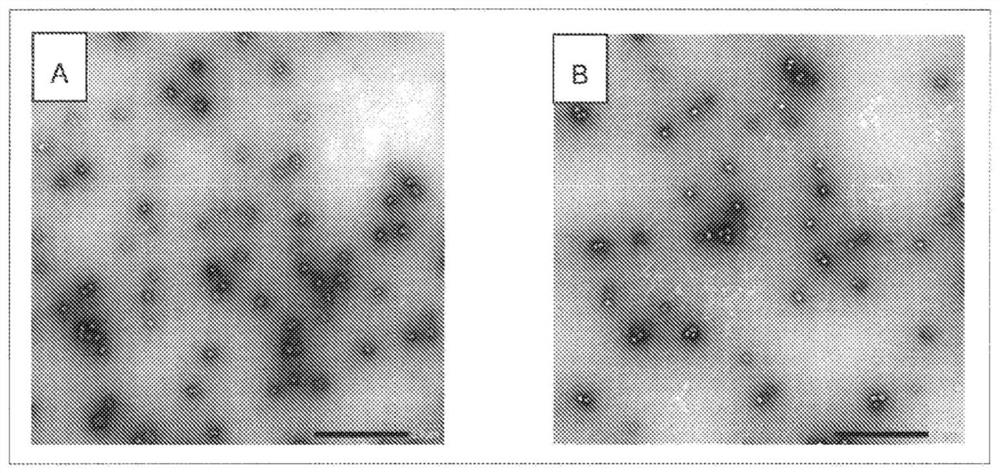
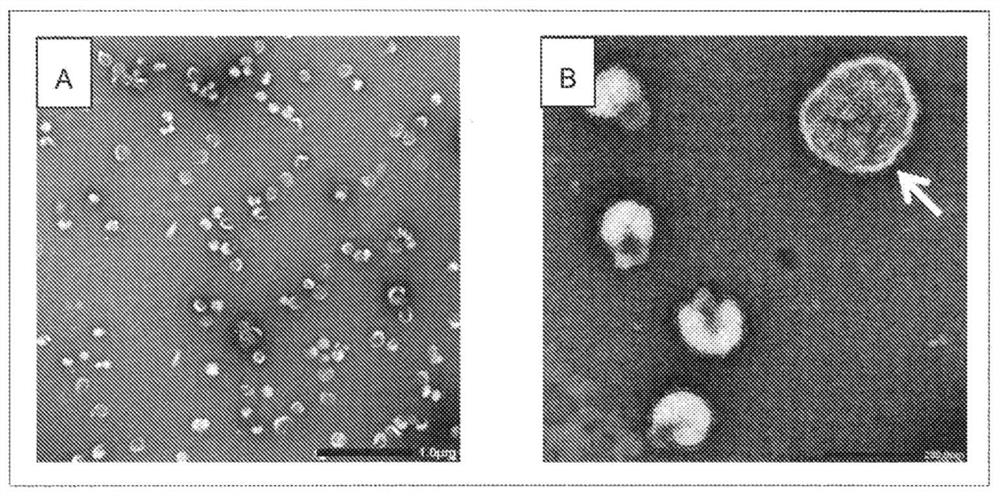
[0054] Reference Example 1 Isolation of extracellular vesicles contained in chorioallantoic fluid of uninfected eggs
[0055] Seventy-two 11-day-old chicken embryos were cooled at 4° C. for 1 hour or more, and chorioallantoic fluid was recovered from each chicken embryo using a disposable needle and syringe (manufactured by Terumo). The recovered chorioallantoic fluid was pooled and centrifuged at 4°C, 300×g for 10 minutes, and the obtained supernatant was centrifuged at 4°C, 141,000×g for 4 hours using an ultracentrifuge (manufactured by Hitachi Koki). The precipitate after ultracentrifugation was suspended in 6.7 mM phosphate-buffered saline (pH 7.2), and centrifuged again at 4° C. and 141,000×g for 4 hours. The obtained pellet was suspended in 6.7 mM phosphate-buffered saline (pH 7.2) to obtain an extracellular vesicle suspension.
[0056] 5 μL of a 0.1% poly-L-lysine solution was dropped onto a TEM electron microscope grid (manufactured by Oken Shoji Co., Ltd.) with a col...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com