Glipizide controlled release tablet and preparation method thereof
A technology of glipizide and controlled-release tablets, which is applied in pharmaceutical formulations, medical preparations of non-active ingredients, drug delivery, etc., can solve the problems of laser intensity attenuation, unstable quality, and high manufacturing costs, and achieve improved solubility , meet the needs of drug administration, and reduce production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
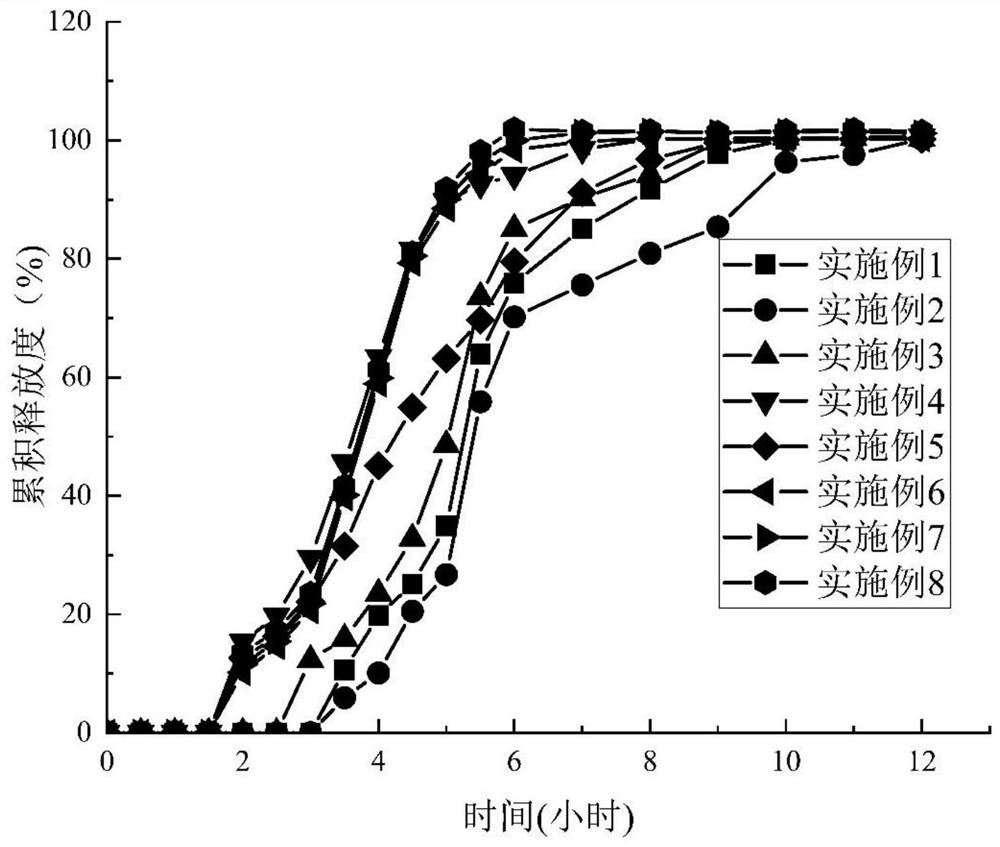
Examples
Embodiment 1
[0054] A kind of preparation method of glipizide controlled-release tablet in the present embodiment comprises the following steps:
[0055] 1) Drug-containing layer
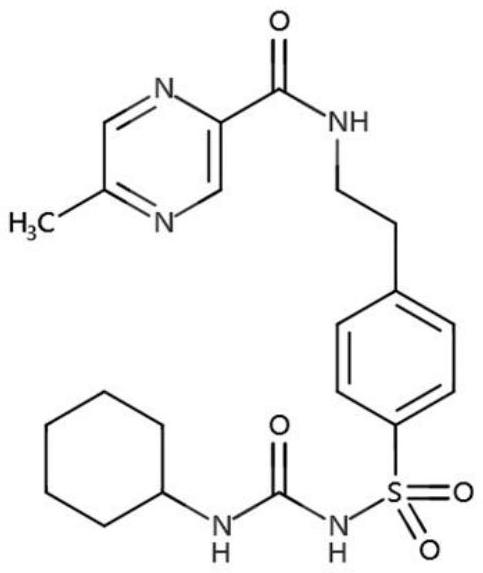
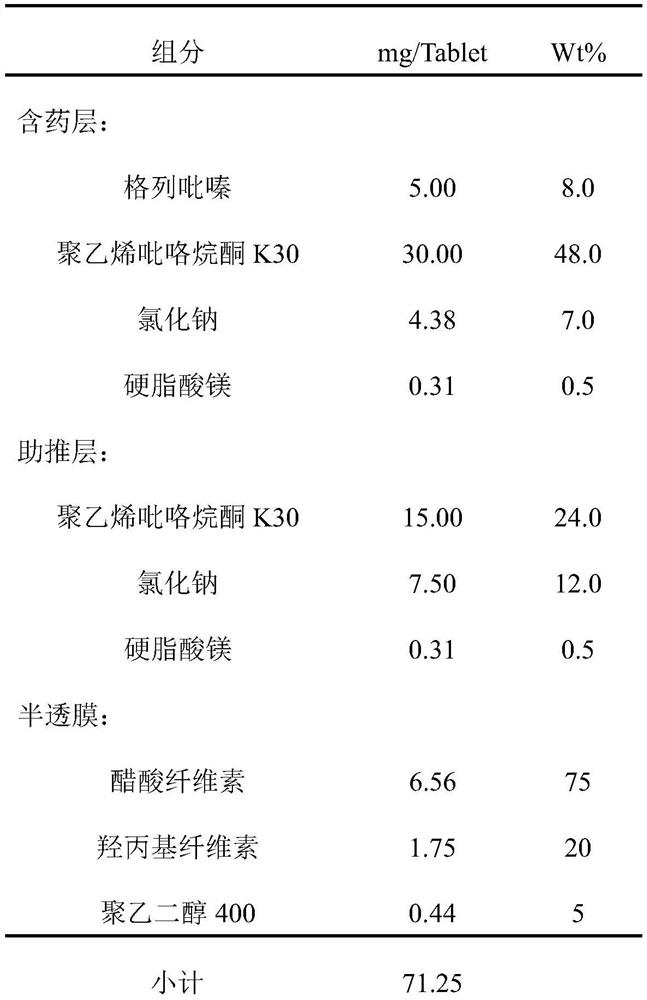
[0056] Put the prescribed amount of polyvinylpyrrolidone K30 and the prescribed amount of glipizide into a low-shear V-type mixer for mixing, mix for 30 minutes to obtain a mixed powder, and then prepare the mixed powder by hot-melt extrusion to prepare a solid dispersion. Crush, sieve, the particle size range is 75-250μm, add the prescribed amount of sodium chloride and magnesium stearate to the prescribed amount of solid dispersion powder, mix in a low-shear V-type mixer, and mix for 30min. The mixed powder of the drug-containing layer is obtained.
[0057] 2) booster layer
[0058] Put the prescribed amount of polyvinylpyrrolidone K30 and sodium chloride into a low-shear V-shaped mixer for mixing, mix for 30 minutes, add the prescribed amount of magnesium stearate, and then mix in a low-shear V-shaped mixer...
Embodiment 2
[0066] A kind of preparation method of glipizide controlled-release tablet in the present embodiment comprises the following steps:
[0067] 1) Drug-containing layer
[0068] Put the prescribed amount of polyvinylpyrrolidone K30 and the prescribed amount of glipizide into a low-shear V-type mixer for mixing, mix for 30 minutes to obtain a mixed powder, and then prepare the mixed powder by hot-melt extrusion to prepare a solid dispersion. Crush, sieve, the particle size range is 75-250μm, add the prescribed amount of hypromellose, sodium chloride and magnesium stearate to the prescribed amount of solid dispersion powder, in a low-shear V-type mixer Mixed for 30 minutes to obtain the mixed powder of the drug-containing layer.
[0069] 2) booster layer
[0070] Put the prescribed amount of polyvinylpyrrolidone K30 and sodium chloride into a low-shear V-shaped mixer for mixing, mix for 30 minutes, add the prescribed amount of magnesium stearate, and then mix in a low-shear V-sha...
Embodiment 3
[0079] A kind of preparation method of glipizide controlled-release tablet in the present embodiment comprises the following steps:
[0080] 1) Drug-containing layer
[0081] Put the prescribed amount of polyvinylpyrrolidone K30 and the prescribed amount of glipizide into a low-shear V-type mixer for mixing, mix for 30 minutes to obtain a mixed powder, and then prepare the mixed powder by hot-melt extrusion to prepare a solid dispersion. Crush, sieve, the particle size range is 75-250μm, add the prescribed amount of hypromellose, sodium chloride and magnesium stearate to the prescribed amount of solid dispersion powder, in a low-shear V-type mixer Mixed for 30 minutes to obtain the mixed powder of the drug-containing layer.
[0082] 2) booster layer
[0083] Put the prescribed amount of polyvinylpyrrolidone K30 and sodium chloride into a low-shear V-shaped mixer for mixing, mix for 30 minutes, add the prescribed amount of magnesium stearate, and then mix in a low-shear V-sha...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com