Fluorine-containing oligomeric silsesquioxane modified hyperbranched copolymer and preparation and application thereof
A technology of polysilsesquioxane and hyperbranched copolymer is applied in the field of preparation of hyperbranched white light copolymer, which can solve the problems of aggregation and quenching, affecting the luminous efficiency and lifespan of the device, and achieves improved performance and improved waterproof and oxygen barrier capability. , good hydrophobicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
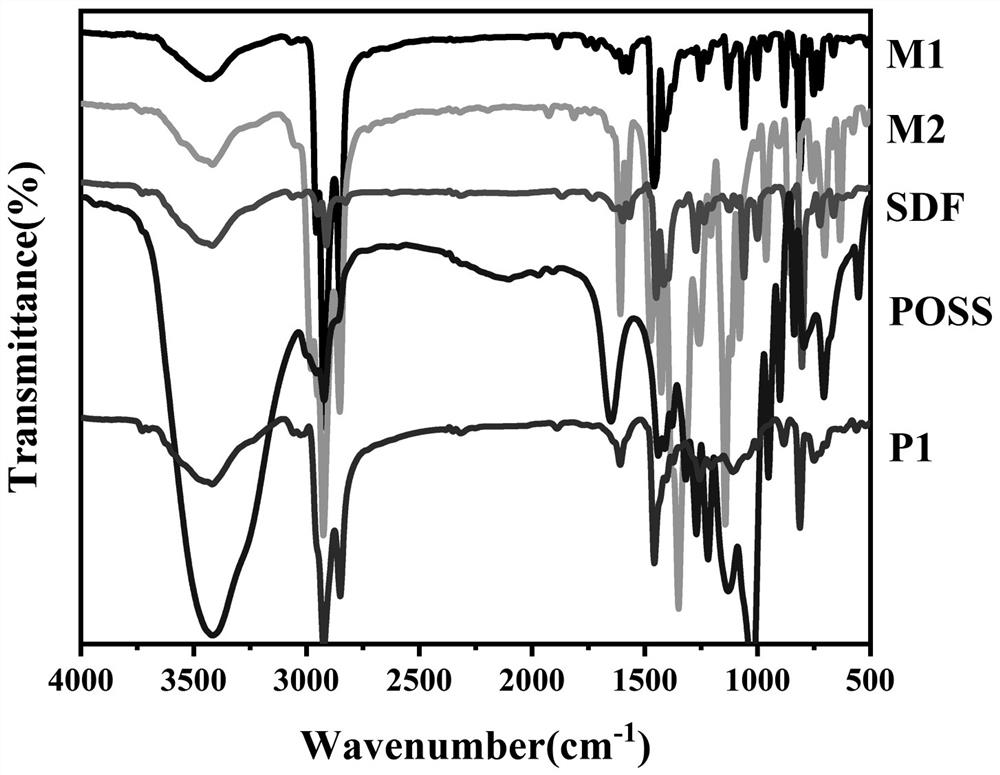
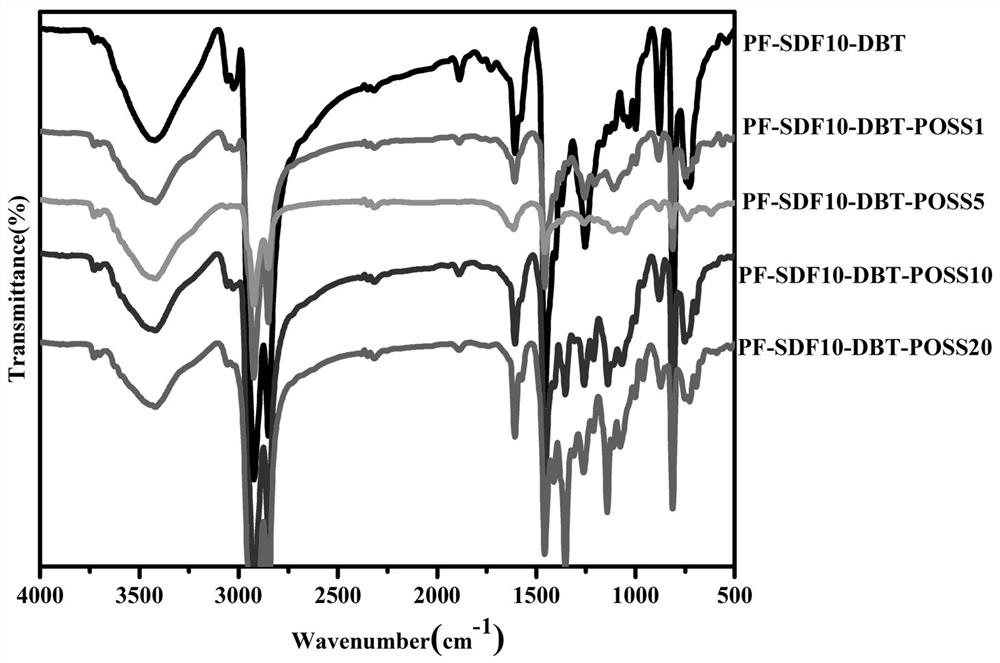
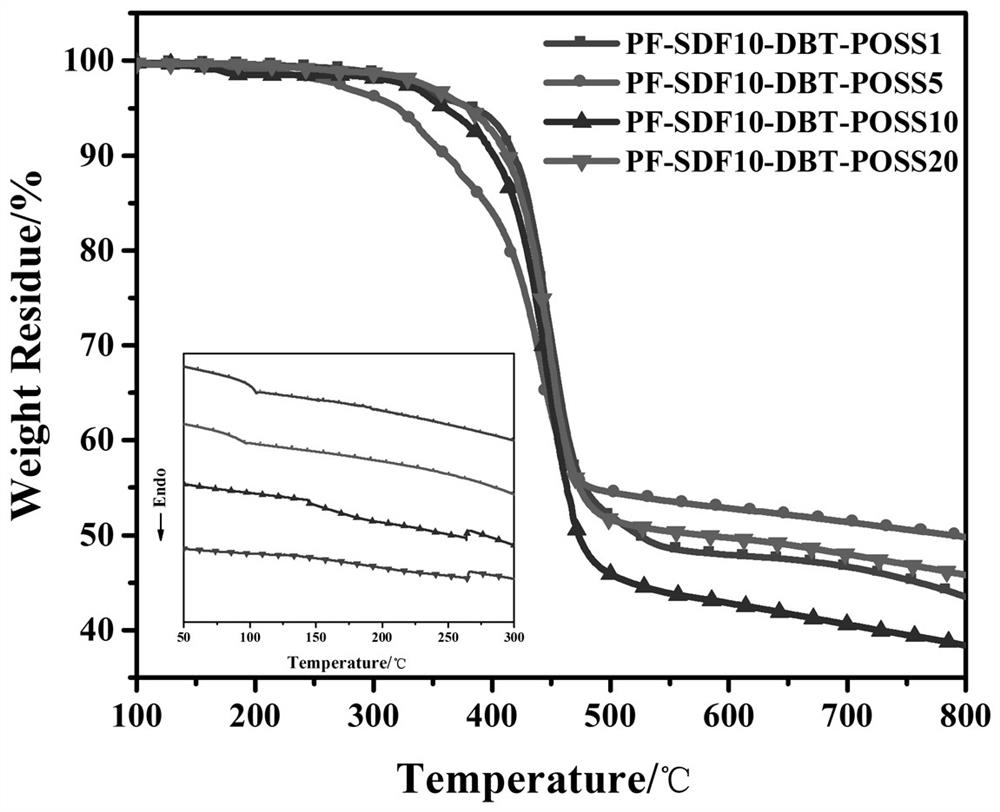
Image
Examples
Embodiment 1
[0060] Example 1: Preparation of fluorine-containing POSS group monomers.
[0061]
[0062] Add 10 g of 3,3,3-trifluoropropyltrimethoxysilane to a two-necked flask previously filled with 50 mL of THF, and vacuum and blow nitrogen.
[0063] Under a nitrogen atmosphere, add 1.05 mL of deionized water into the two-necked flask, raise the temperature to 50°C and stir for 30 minutes, then add 790 mg of NaOH, raise the temperature to 80°C, stir and reflux for 5 hours. Heating was stopped, and after stirring at room temperature for 15 h, the reaction ended.
[0064] The reaction product was concentrated under reduced pressure to remove the solvent and other volatiles, and dried in a vacuum oven at 40° C. for 12 hours to obtain a white powder of the fluorine-containing POSS group precursor.
[0065]
[0066] Weigh 10 g of fluorine-containing POSS precursor into a two-neck flask, vacuumize and ventilate nitrogen, add 80 mL of anhydrous THF under nitrogen atmosphere and vigorous ...
Embodiment 2
[0069] Example 2: Preparation of 2-(2-bromoethoxy)tetrahydro-2H-pyran.
[0070]
[0071] Add p-toluenesulfonic acid hydrate (PPTS) (6.36g, 36mmol) into a three-necked flask, add 80mL solvent dichloromethane, vacuumize the reaction system, and blow nitrogen three times.
[0072] Continue to inject 3,4-dihydro-2H-pyran (34.8mL, 386mmol) and 2-bromoethanol (18mL, 254mmol) into the three-necked flask, and stir at room temperature for 12h.
[0073] After the mixture was cooled, it was washed with ethyl acetate and saturated NaCl aqueous solution, and the organic phase was washed with anhydrous MgSO 4 Drying, filtration, vacuum concentration of the filtrate, silica gel chromatographic column, purification with petroleum ether:dichloromethane=4:1 as the eluent, to obtain the target product 2-(2-bromoethoxy) in the form of light yellow viscous liquid Tetrahydro-2H-pyran.
Embodiment 3
[0074] Example 3: Preparation of 2-{2-[2,7-dibromo-9-(2-perhydro-2H-pyran-2-yloxyethyl)fluoren-9-yl]ethoxy}all Hydrogen-2H-pyran.
[0075]
[0076] Add 2,7-dibromofluorene (5g, 15mmol) and phase transfer catalyst tetrabutylammonium chloride (TBAcl) (0.42g, 1.5mmol) into a three-necked flask, add 60mL DMSO, vacuumize and pass nitrogen 3 times .
[0077] Raise the temperature of the system to 55°C, add 20mL of 50wt% NaOH solution, stir mechanically for 5min, then add 2-(2-bromoethoxy)tetrahydro-2H-pyran (10.5g, 50mmol), raise the temperature to 65°C, The reaction was stirred for 12h.
[0078] After cooling, wash with ethyl acetate and saturated NaCl aqueous solution, then wash with deionized water three times, anhydrous MgSO 4 After drying, filtering, and concentrating the filtrate in vacuum, it was applied to a silica gel chromatography column and purified with petroleum ether:ethyl acetate=10:1 as the eluent to obtain the target product 2-{2-[2,7- Dibromo-9-(2-perhydro-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| water contact angle | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
| surface roughness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com