Method for enhancing synthesis ammonia selectivity by ball-milling nitrogen-hydrogen mixed gas at normal temperature and normal pressure in water phase
A nitrogen-hydrogen mixing and ammonia synthesis technology, applied in the field of materials, can solve problems such as environmental pollution, large energy consumption, and high equipment requirements, and achieve the effects of improving selectivity, increasing sources and concentrations, and enhancing performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
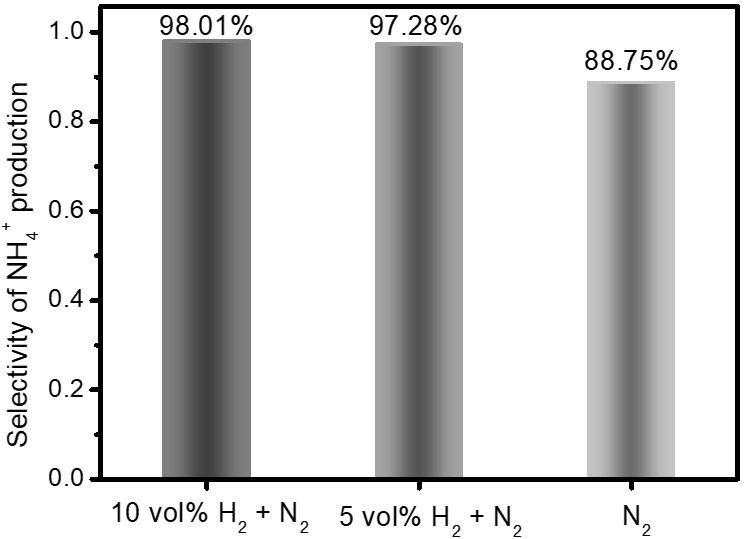
[0019] (1) Put 50mL of pure water and 300g of stainless steel balls of different sizes in a stainless steel vacuum ball milling jar with a capacity of 250mL, and inject nitrogen-hydrogen mixture gas (10 vol% H 2 + 90vol%N 2 ) for 30 minutes, get rid of the air in the tank, seal the ball mill tank so that the tank is in an environment full of nitrogen-hydrogen mixture at normal temperature and pressure, and the volume of the mixture is 180mL;
[0020] (2) Install the ball mill jar prepared in step (1) in a planetary ball mill, mill at a rate of 600 rpm for 10 hours, and take out the ball mill jar after the reaction is completed;
[0021] (3) Take out the solution in the tank in step (2), let it stand still, take the supernatant, and centrifuge twice through the centrifuge to further remove the iron filings generated during the ball milling process, each time at 15000r / min, 5min;
[0022] (4) Detect the aqueous solution in step (3) by ion chromatography, and measure the NH in ...
Embodiment 2
[0025] (1) Put 50mL of pure water and 300g of stainless steel balls of different sizes in a stainless steel vacuum ball milling jar with a volume of 250mL, and inject a nitrogen-hydrogen mixture (5vol% H 2 + 95vol%N 2 ) for 30 minutes, get rid of the air in the tank, seal the ball mill tank so that the tank is in an environment full of nitrogen-hydrogen mixture at normal temperature and pressure, and the volume of the mixture is 180mL;
[0026] (2) Install the ball mill jar prepared in step (1) in a planetary ball mill, mill at a rate of 600 rpm for 10 hours, and take out the ball mill jar after the reaction is completed;
[0027] (3) Take out the solution in the tank in step (2), let it stand still, take the supernatant, and centrifuge twice through the centrifuge to further remove the iron filings generated during the ball milling process, each time at 14000r / min, 7min;
[0028] (4) Detect the aqueous solution in step (3) by ion chromatography, and measure the NH in the so...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com