Porcine rotavirus recombinant protein, recombinant adenovirus expressing same protein and application
A porcine rotavirus, recombinant adenovirus technology, applied in applications, viruses, viral peptides, etc., can solve the problems of no human adenovirus vector, low probability of repeated use of vaccines, and achieve low dosage and high yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] This embodiment provides a method for constructing a recombinant adenovirus, the specific process is as follows:
[0036] 1. Construction of transfer vector pShuttle-CMV-G5VP7-G9VP8
[0037] 1.1 Obtaining the target genes PoRV-VP7 and VP8
[0038] According to the codon preference of adenovirus expression system, the nucleotide sequences of porcine rotavirus type 5 VP7 protein and type 9 VP8 protein were codon optimized. Gene synthesis was performed by Sangon Bioengineering Co., Ltd. The synthetic gene sequence is shown in SEQ ID NO: 1 (also contains an IL-2 signal peptide at the beginning). The synthetic target gene is placed on the plasmid vector pUC-SP, which is obtained in the form of pUC-SP-G5VP7-G9VP8.
[0039] 1.2 Construction of recombinant transfer vector pShuttle-CMV-PoRV-G5VP7-G9VP8
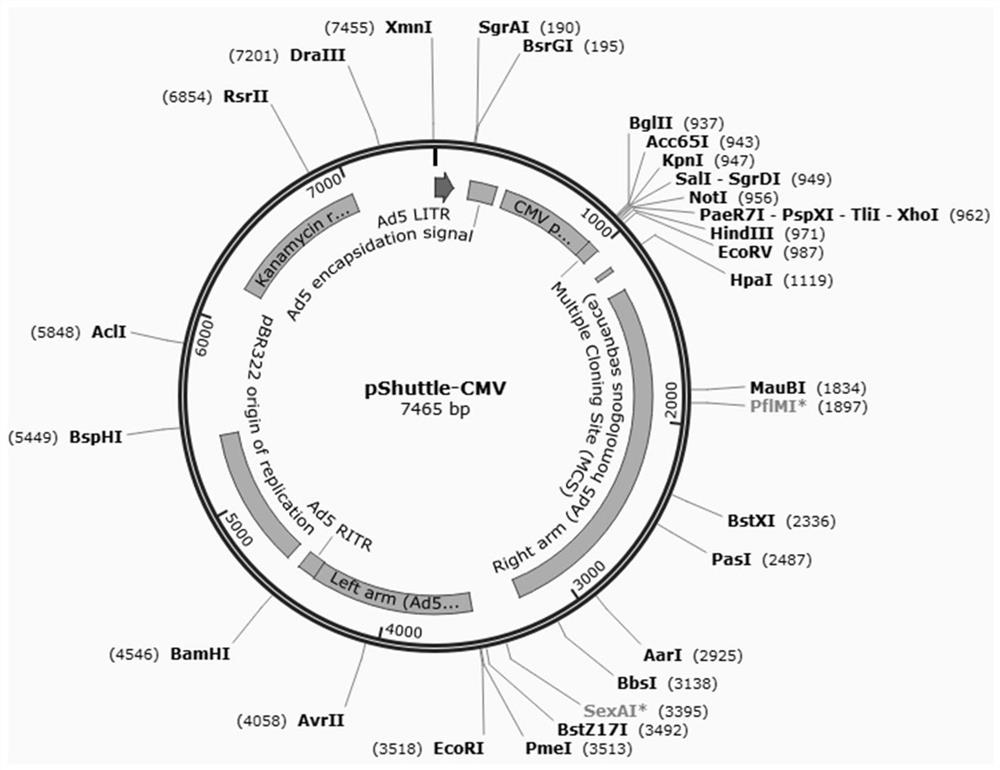
[0040] Insert the G5-VP7 and G9-VP8 genes of PoRV into the pShuttle-CMV vector by recombination, see the attached map figure 1 :
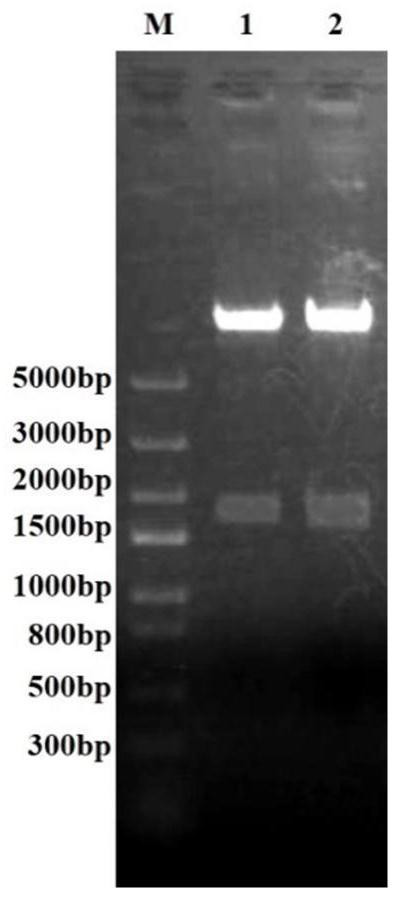
[0041] (1) PCR amplification: through PC...
Embodiment 2
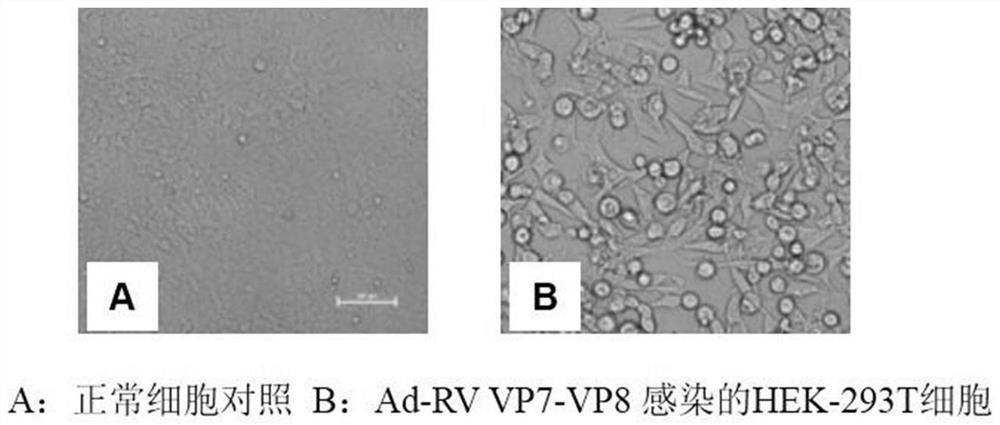
[0070] In this example, the recombinant adenovirus prepared in Example 1 was used to prepare a vaccine, and its performance was tested. The process is as follows:
[0071] 1. Evaluation of adenovirus Ad-PoRV-G5VP7-G9VP8 vector vaccine in pigs
[0072] 1.1 Safety evaluation of adenovirus Ad-PoRV-G5VP7-G9VP8 vector vaccine in pigs: 8 experimental pigs (PoRV-negative pigs aged 14-21 days) were purchased and divided into two groups, A and B, with 4 pigs in each group . Group A intramuscular injection of 10 per head and neck 10 TCID 50 Recombinant adenovirus (1mL); group B was intramuscularly injected with 1mL sterilized PBS on the head and neck, and observed continuously for 14 days. The pigs in the two groups were in the same state and had no abnormal reaction, which indicated that the vaccine was safe for pigs.
[0073] 1.2 Immunogenicity evaluation of adenovirus Ad-PoRV-G5VP7-G9VP8 vector vaccine in pigs: 12 experimental pigs were purchased and randomly divided into three gr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com