Iron-based perovskite mixed conductor oxygen permeation membrane material for hydrogen production by thermochemical decomposition of water and preparation method thereof
A mixed conductor and perovskite technology, applied in the field of ceramic manufacturing, can solve problems such as poor stability, corrosion of oxygen permeable film, and difficulty in long-term operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
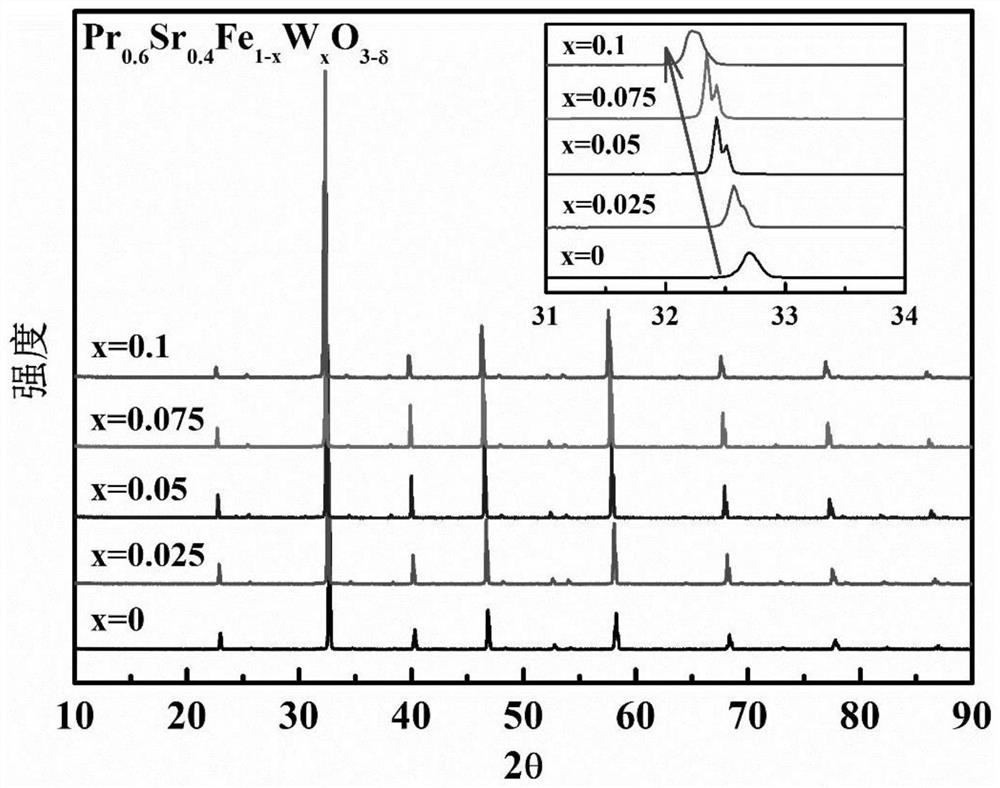
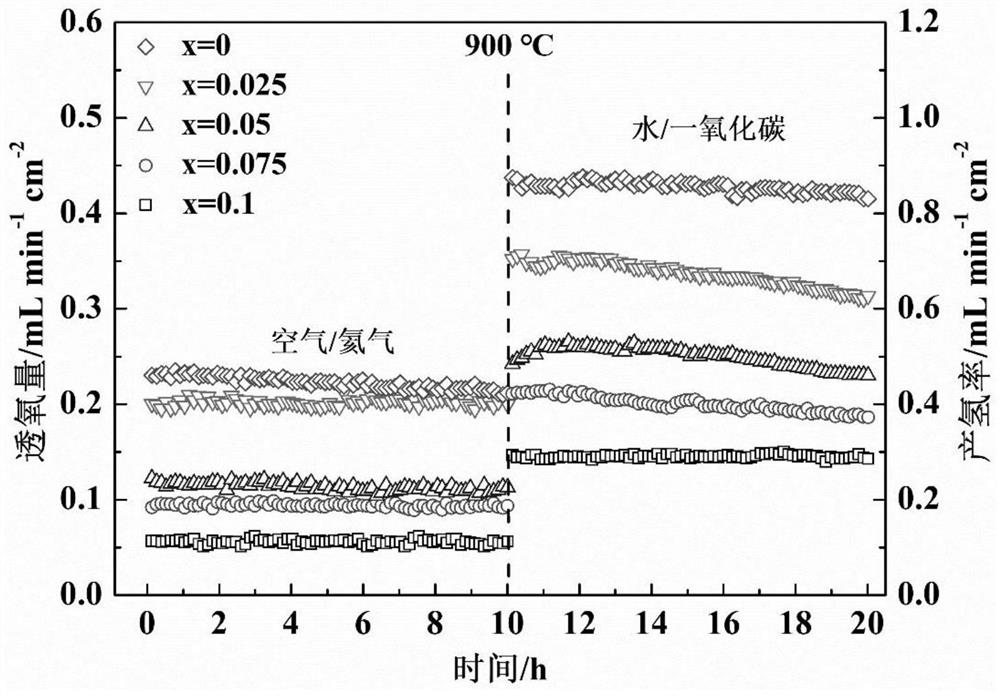
[0029]In the present embodiment, the thermochemical decomposing of the iron-based calcium ore mixed conductor oxygenated membrane material with the following composition:
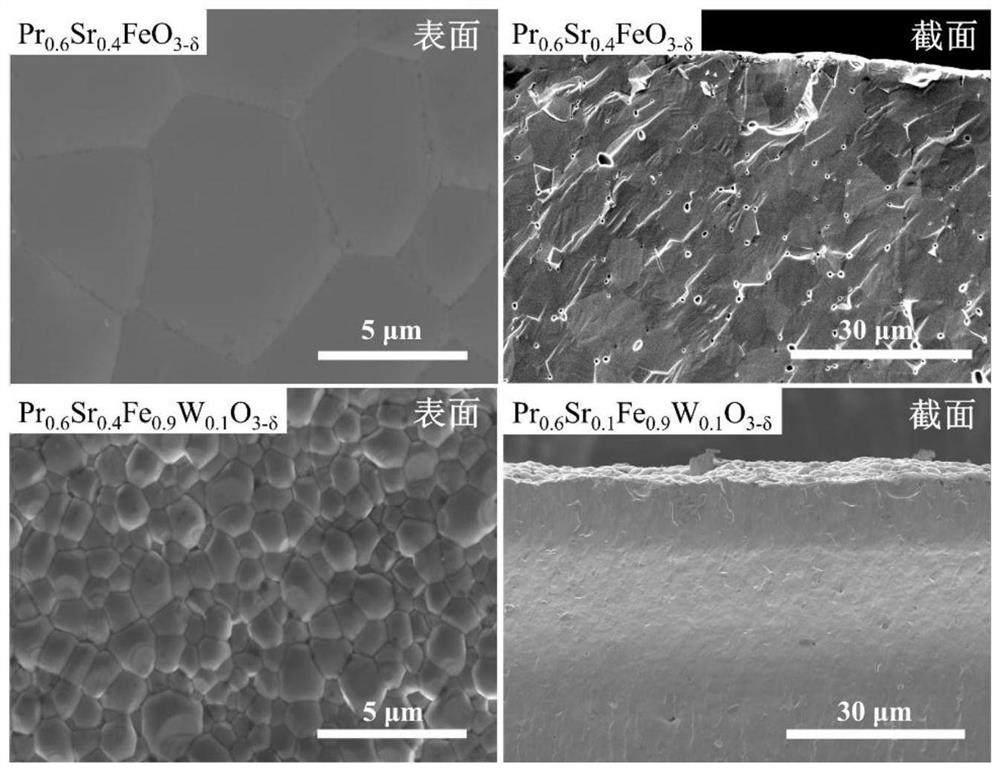
[0030]PR0.6SR0.4Feo3-Δ .
[0031]In the present embodiment, thermochemical decomposing the preparation method of ferriths titanium hydrogen mixing conductor oxygenate material, including the following steps:
[0032]23.36g Pr (NO3)3· 6h2O, 7.58G SR (NO3)2, 36.16g Fe (NO3)3· 9h2O dissolve in deionized water; 52.36 g of ethylenediamine tetracetic acid and 56.43 g of citric acid dissolved in another beaker equipped with deionized water, mix the above two solutions, stirred, and regulate the solution by dripping ammonia water. The pH was 8, continued to 80 ° C and stir until the sol was obtained. The resulting sol was dried at 150 ° C until it was taken out after the sponge porous solid was taken out, and after 10 hours at 350 ° C, the obtained soil brown powder was ground after 5 hours at 950 ° C for 5 hours, that is, the PR...
Embodiment 2
[0037]This embodiment is substantially the same as the examples, in particular
[0038]In the present embodiment, the thermochemical decomposing of the iron-based calcium ore mixed conductor oxygenated membrane material with the following composition:
[0039]PR0.6SR0.4FE0.9W0.1O3-Δ .
[0040]In the present embodiment, thermochemical decomposing the preparation method of ferriths titanium hydrogen mixing conductor oxygenate material, including the following steps:
[0041]22.10g Pr (NO3)3· 6h2O, 7.17G SR (NO3)2, 30.78g Fe (NO3)3· 9h2O Dissolve in deionized water, 1.96GWO3The mixture dissolved in ammonia was dissolved in ammonia, and the mixed solution of metal ions was mixed; 49.48 g of ethylenediamine tetracetic acid and 53.37 g of citric acid were dissolved in another beaker equipped with deionized water, and the above two solutions were mixed. Heating and stirring, the pH of the solution was 80 ° C and stirred until it was allowed to be heated to 80 ° C and stirred until the sol was allowed ...
Embodiment 3
[0046]This embodiment is substantially the same as the foregoing embodiment, in particular
[0047]In the present embodiment, the thermochemical decomposing of the iron-based calcium ore mixed conductor oxygenated membrane material with the following composition:
[0048]PR0.6SR0.4FE0.9V0.1O3-Δ .
[0049]In the present embodiment, thermochemical decomposing the preparation method of ferriths titanium hydrogen mixing conductor oxygenate material, including the following steps:
[0050]23.41g Pr (NO3)3· 6h2O, 7.59g SR (NO3)232.62G Fe (NO3)3· 9h2O Dissolve in deionized water, 1.63GV2O5The mixture dissolved in ammonia is mixed in ammonia and mixed with metal ions; the mixture of 52.43 g of ethylenediamine tetracetic acid and 56.55 g of citric acid were dissolved in another beaker equipped with deionized water, and the above two solutions were mixed. The mixture was heated, and the pH of the solution was 80 ° C and stirred until it was heated to 80 ° C and stirred until it was added to 80 ° C. The r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com