Germ model screening system and screening method
A model and screening technology, applied in biological systems, analytical materials, biological material analysis, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] Embodiment 1 screening system
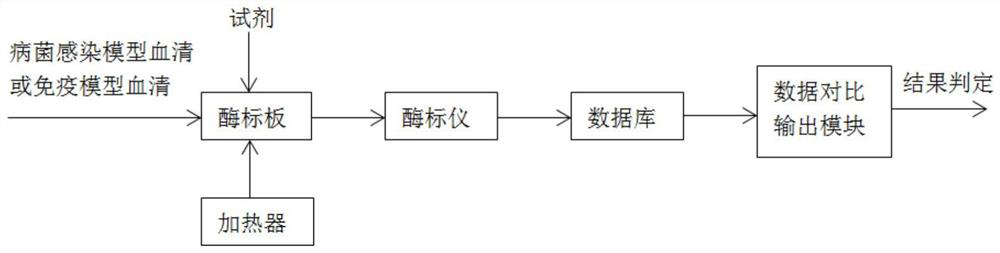
[0106] refer to figure 1 , a system for screening infectious bacteria models, including microplates, heaters, reagents, databases, microplate readers, and data comparison output modules;
[0107] Among them, the enzyme label plate includes polystyrene / polyvinyl chloride material standard plate, including 96 holes or 48 holes;
[0108] Among them, the reagents include antigen coating solution, enzyme-labeled antibody, chromogenic solution, bacterial infection model serum or immune model serum;
[0109] Among them, the database produces the absorbance of different antibody amounts for healthy or infected or immune animal models, which can be imported and deleted;
[0110] Wherein, the microplate reader is set to have a wavelength of 450nm or 410nm.
[0111] Wherein, the chromogenic solution is TMB single-component chromogenic solution.
[0112] Wherein, the antigen coating solution includes PLO coating solution with a concentration of 0...
Embodiment 2
[0113] Example 2 Bacterial Culture and Preparation of Recombinant Protein
[0114] (1) Bacterial culture
[0115] Inoculate a single colony of Cryptobacterium pyogenes (biological deposit number: CCTCC NO: M2014037, GenBank NO: CP012649) in TSB medium containing 8% calf serum, and shake at 37°C for 48 hours;
[0116] A single colony of Corynebacterium pseudotuberculosis was inoculated in TSB medium, and shaken at 37°C for 24 hours;
[0117] Single colonies of Staphylococcus aureus, Proteus mirabilis, and Escherichia coli were inoculated in LB medium respectively, and cultured with shaking at 37°C overnight.
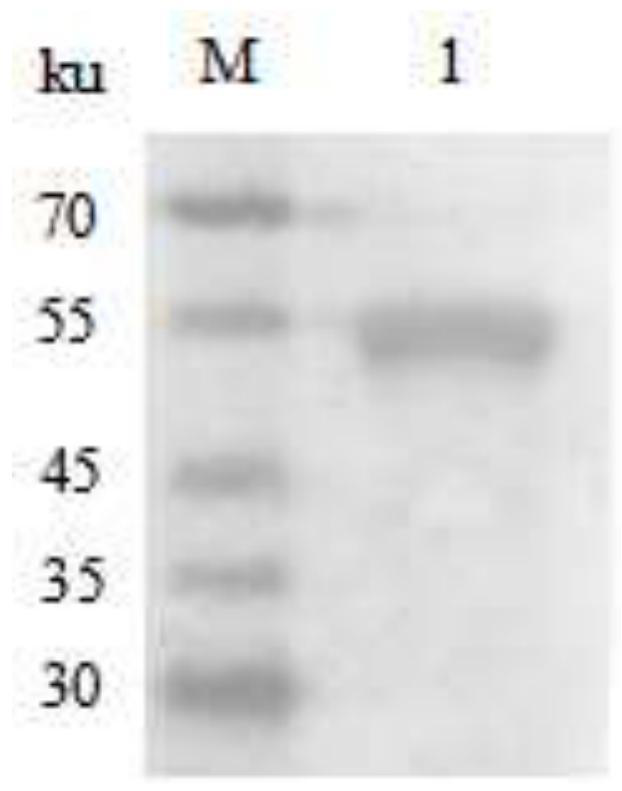
[0118] (2) Identification of Cryptobacter pyogenes PLO
[0119] The C. pyogenes freshly cultured in Example 1 was centrifuged at 6 000 r / min for 10 min, and the supernatant was retained. The supernatant was filtered through a 0.22 μm filter membrane and concentrated by ultrafiltration with a molecular cut-off of 10,000ku. The PLO in the concentrated solution was ident...
Embodiment 3
[0135] Embodiment 3 test analysis
[0136] (1) Cross immune protection test
[0137] The inactivated vaccine of C. pyogenes made with aluminum gel adjuvant was used to immunize mice according to the method of step (4) in Example 2, and a total of 45 mice were immunized. Two weeks after the third immunization, the immunized mice were intraperitoneally inoculated with a lethal dose of Corynebacterium pseudotuberculosis, and 15 unimmunized healthy mice were inoculated intraperitoneally with a lethal dose of Corynebacterium pseudotuberculosis. The death rate of the mice was counted.
[0138] Two weeks after the third inactivated C. pyogenes vaccine, a lethal dose of Corynebacterium pseudotuberculosis was inoculated intraperitoneally. The survival rate of the immunized mice was 53.3% (24 / 45), and one unimmunized mouse survived. This indicated that inactivated C. pyogenes immunization had a cross-immune protective effect on Corynebacterium pseudotuberculosis.
[0139] (2) Agglutina...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
| Absorbance values | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com