Stable NSE quality control product and preparation method thereof
A technology of quality control products and human serum, applied in the biological field, can solve the problems of inconvenient detection, decreased NSE level, poor reconstitution stability, etc., and achieve the effects of high accuracy, reduced decline speed, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Effect of magnesium ions on the liquid stability of NSE compared with other ions
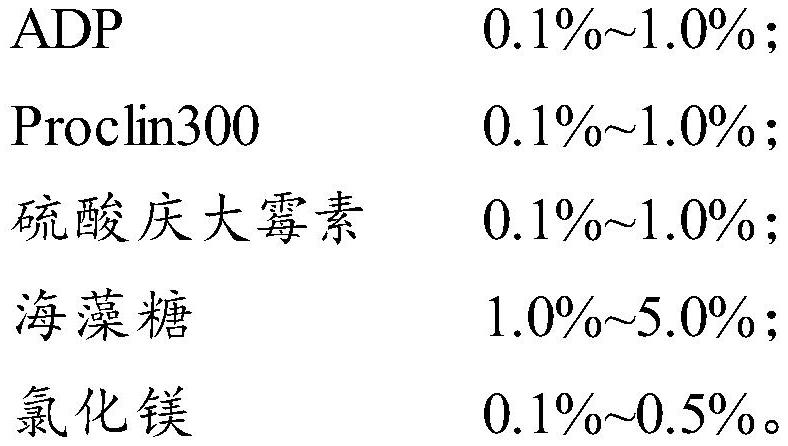
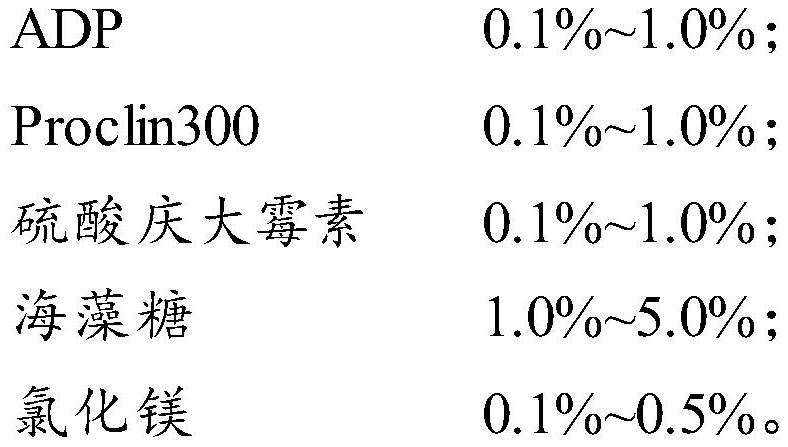
[0033] In the serum of a healthy person, add ADP to a mass fraction of 0.3%, add Proclin300 to a mass fraction of 0.2%, add gentamicin sulfate to a mass fraction of 0.1%, add trehalose to a mass fraction of 3%, and stir thoroughly Mix well until the matrix solution is clear, place it at 2-8°C, add NSE antigen (from Zhengzhou Yimenuo Biotechnology Co., Ltd.), divide the solution into three levels according to the amount of NSE antigen added, and the concentration of NSE antigen is respectively 20ng / mL, 60ng / mL, 120ng / mL.
[0034] Each level of antigen-containing solution is divided into four groups, a total of 12 groups, adding sodium ions (to a mass fraction of 0.2%), magnesium ions (to a mass fraction of 0.2%), iron ions (to a mass fraction of 0.2%) and 12 groups in total. ) and copper ions (to a mass fraction of 0.2%) were prepared into 12 kinds of NSE quality control products.
[003...
Embodiment 2
[0040] To verify the effect of magnesium ions on the stability of NES quality control (liquid).
[0041] In the serum of a healthy person, add ADP to a mass fraction of 0.3%, add Proclin300 to a mass fraction of 0.2%, add gentamicin sulfate to a mass fraction of 0.1%, add trehalose to a mass fraction of 3%, and stir thoroughly Mix well until the matrix solution is clear, place it at 2-8°C, add NSE antigen (from Zhengzhou Yimenuo Biotechnology Co., Ltd.), divide the solution into three levels according to the amount of NSE antigen added, and the concentration of NSE antigen is respectively 20ng / mL, 55ng / mL, 100ng / mL.
[0042] The solutions containing antigens at each level were divided into 2 groups (with or without magnesium ions), a total of 6 groups, and were prepared into 12 kinds of NSE quality control products.
[0043] Two replicates were set up for each quality control product, and the liquid state was stored at 4°C for 1d, 2d, 3d, 4d, 5d, 6d, and 7d, and the concentra...
Embodiment 3
[0048] To verify the effect of magnesium ions on the stability of NES quality control (lyophilized).
[0049] In the serum of healthy people, add ADP to a mass fraction of 0.3%, add Proclin300 to a mass fraction of 0.2%, add gentamicin sulfate to a mass fraction of 0.1%, add trehalose to a mass fraction of 3%, and then Add magnesium chloride to a mass fraction of 0.2%, stir and mix well until the matrix solution is clear, place it at 2-8°C, add NSE antigen (from Zhengzhou Yimenuo Biotechnology Co., Ltd.), and divide the solution according to the amount of NSE antigen added. There are three levels, in which the concentration of NSE antigen is 20ng / mL, 55ng / mL, 100ng / mL respectively.
[0050]The NSE quality control product prepared into three levels is subpackaged and freeze-dried. The freeze-drying procedure is: primary drying (sublimation drying) and secondary drying (desorption drying). Specific freeze-drying parameters:
[0051] During the cooling process, the temperature d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com