Aspirin and clopidogrel hydrogen sulfate compound preparation and preparation method thereof
A compound formulation of clopidogrel hydrogen sulfate and a technology of clopidogrel hydrogen sulfate are applied in the field of medicine and can solve the problems of blockage, high growth of salicylic acid, precipitation of talc and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-3
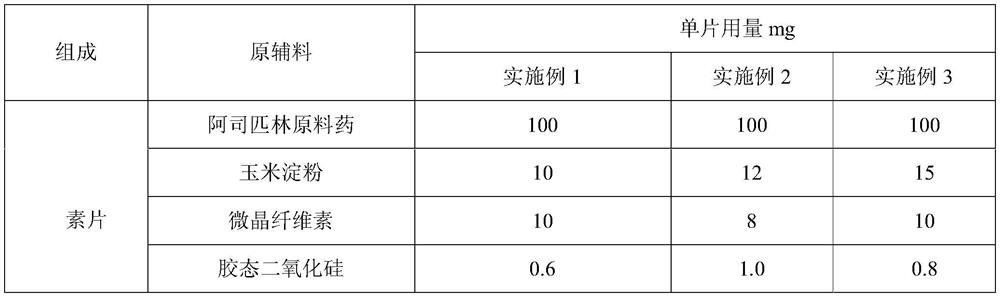
[0050] The preparation of embodiment 1-3 aspirin tablet
[0051] Table 1: Preparation prescription of aspirin tablet
[0052]
[0053] Preparation Process:
[0054] 1. After crushing aspirin with a hammer mill at 2000rpm, pass through a 1.0mm sieve and add cornstarch into a three-dimensional motion mixer for 30min at 25Hz;
[0055] 2. Put the mixed material into the dry granulator, the feeding speed is 25-50r / min, the pressure wheel speed is 10-25r / min, the oil pressure is 5-8MPa, the crushing speed is 120r / min, and the pre-sizing sieve is 8 mesh , the pre-sizing speed is 60-80r / min, the final sizing sieve is 20 mesh, and the final sizing speed is 60-80r / min. The prepared granules are sieved with a vibrating sieve (20 mesh, 80 mesh), and the granules between the 20-80 mesh sieves are collected.
[0056] 3. Add the prepared aspirin starch granules, microcrystalline cellulose and colloidal silicon dioxide into a three-dimensional motion mixer and mix for 20 minutes at 25 H...
Embodiment 4-8
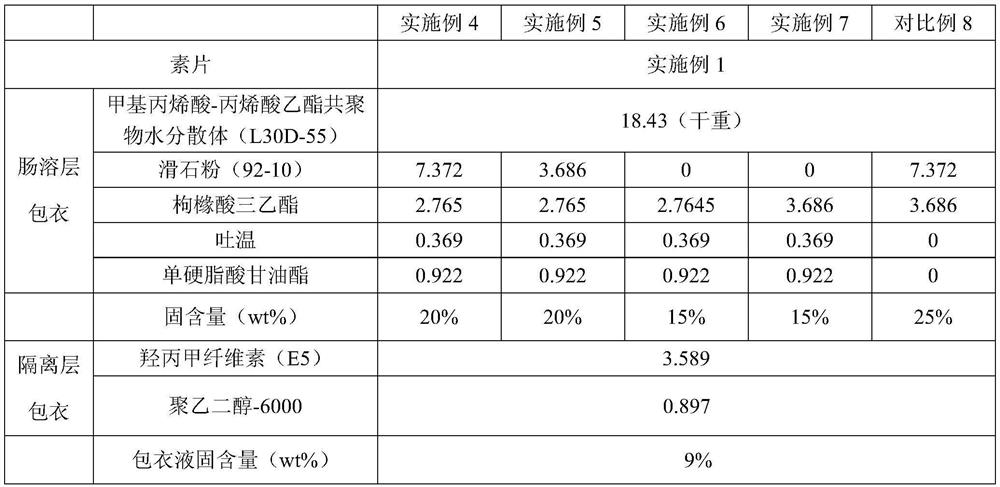
[0058] Embodiment 4-8: Preparation of aspirin enteric-coated tablet core
[0059] Table 2: Preparation recipe of aspirin enteric-coated tablet core
[0060]
[0061]
[0062] Preparation Process:
[0063] 1. Enteric layer coating:
Embodiment 4-5
[0065] Coating solution configuration:
[0066] A: Add the prescribed amount of Tween 80 and 1 / 2 the amount of triethyl citrate and glyceryl monostearate to an appropriate amount of hot purified water at 70-80°C to prepare a solution with a solid content of 15%, stir and dissolve Homogeneously emulsify and disperse, add the remaining purified water (after removing the hot water in configuration A and purified water in configuration B) after emulsification, stir evenly and cool down to below 30°C;
[0067] B: Add the remaining 1 / 2 amount of triethyl citrate and talcum powder to an appropriate amount of purified water for homogenization and emulsification for 5-10 minutes to make a solution with a solid content of 20%;
[0068] Add the prepared solution A into solution B under stirring, add the suspension into Eudragit L30D aqueous dispersion (solid content 30wt%) under stirring, continue stirring for 1 hour and pass through a 60-mesh sieve.
[0069] Coating parameters: Put the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com