Energy-saving method for preparing amine compound by continuous hydrogenation of nitrile compound
An amine compound and compound technology, which is applied in the field of energy-saving nitrile compound continuous hydrogenation to prepare amine compound, can solve the problems of reduced catalyst life, reduced production cost, reduced selectivity and catalyst life, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
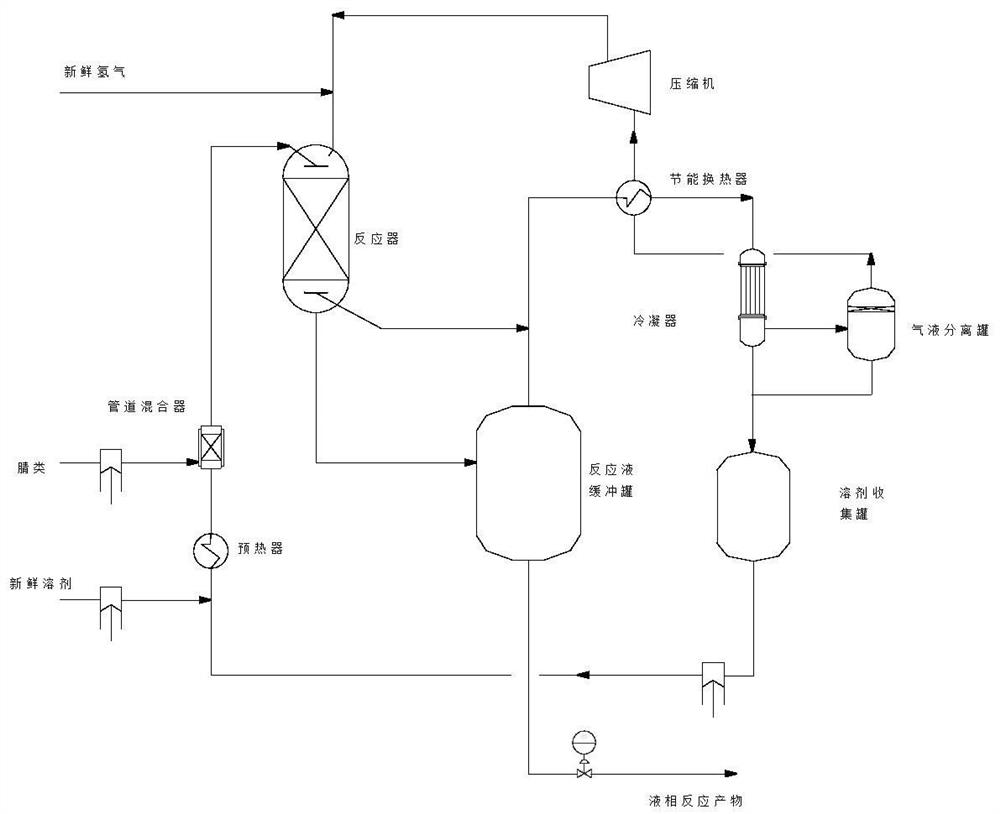
Image
Examples
Embodiment 1
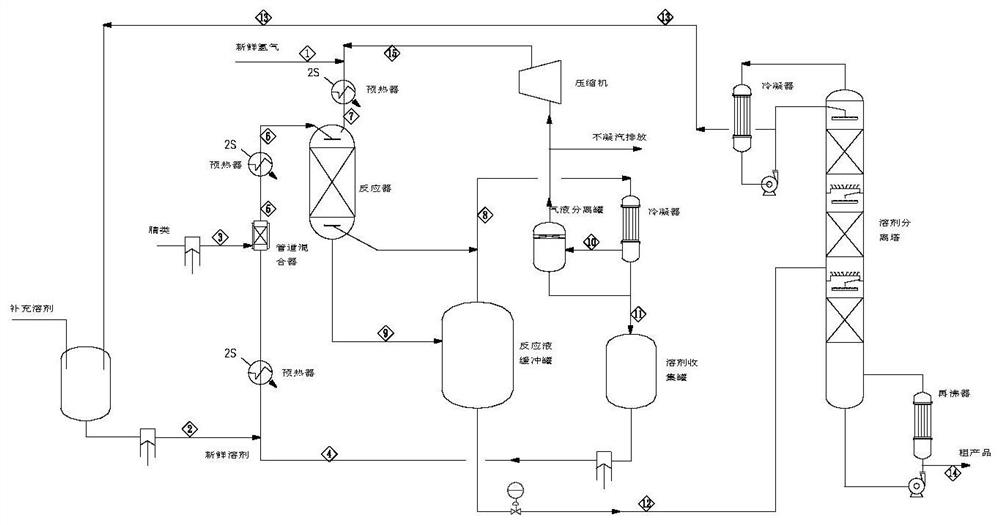
[0079] Prepare m-xylylenediamine (MXDA) by continuous hydrogenation of isophthalonitrile (MXPN), and its flow process of the preparation method adopted is as follows image 3 As shown, the marks 1-15 marked in the figure are the numbers of material streams.
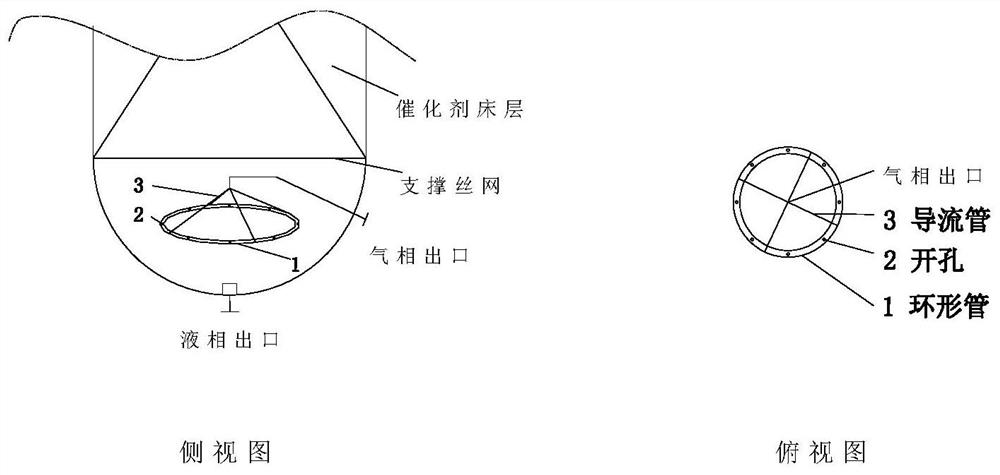
[0080] The size of a single trickle bed reactor is D=0.1m, TL=2m. Catalyst (cat-1) is randomly packed in the upper section of the reactor, and the upper and lower ends are piled up with ceramic balls at a height of 0.3m to prevent the loss of the catalyst, and then the catalyst and the ceramic balls are fixed in the straight section with wire mesh. Two multi-point multi-branch thermocouple temperature probes are evenly inserted into the reactor bed to measure the temperature change of the hot spot of the bed in real time. An annular tube gas collector is installed horizontally in the lower section of the reactor, and the diameter of the annular tube is calculated as Φ15mm according to the pressure drop of 100 meters not ...
Embodiment 2
[0125] The preparation method refers to Example 1, the only difference is that the catalyst cat-1 is replaced by cat-3, the raw material is still MXPN, the solvent is liquid ammonia, the feed ratio, feed space velocity, cyanamide ratio, reaction temperature and pressure Consistent with Example 1, the reactor has a volume of 100ml, a length of 1m, an inner diameter of 20mm, and a catalyst loading capacity of 50ml. The feed rate of raw materials is 375ml / h, and the results of running for 840h are shown in Table 8. It can be found that when magnesium oxide is not added to the catalyst, the catalyst activity is relatively stable for the first 504 hours, but as the reaction time continues, the surface of the catalyst is gradually attached by heavy components, and the catalyst activity gradually decreases, resulting in an increase in aminonitriles, and the overhydrogenation product 1.3 cyclohexyl The gradual increase of the dimethylamine content shows that the specificity of the cat...
Embodiment 3
[0130] The preparation method refers to Example 1, the only difference is that the catalyst cat-1 is replaced by cat-5. The reaction conditions such as feed composition and ratio, space velocity, cyanamide ratio, reaction temperature and pressure are consistent with those in Example 1. The reactor has a volume of 100ml, a length of 1m, an inner diameter of 20mm, and a catalyst loading of 50ml. The raw material feed rate is 375ml / h, and the results of running for 72 hours are shown in Table 9. It can be found that compared with Example 1, the conversion rate of MXPN has no significant change, but the product selectivity decreases significantly, and the by-product benzylamine light components (comprising 1.3-cyclohexanedimethylamine, methylbenzylamine, benzylamine, etc.) ) significantly increased, and by-product amide heavy components such as dicondensate and tricondensate increased significantly.
[0131] Table 9
[0132]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com