Design of activated T cells of bispecific T cell activator and application thereof
A technology of cells and cell groups, applied in the direction of receptor/cell surface antigen/cell surface determinant, for targeting specific cell fusion, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, etc., can Solve the problems of neurotoxicity and CAR-T therapy not showing clinical efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
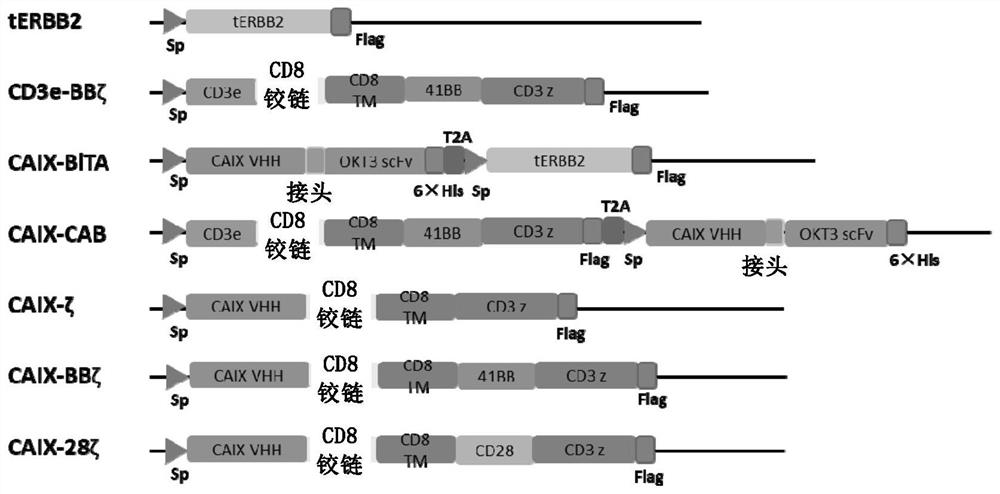
[0177] The design of embodiment 1CAB and contrast structure
[0178] 1.1 Control group CD3e-BBζ, 1 st -CAIX-BiTA, 1 st - Structure design of CAIX-CAB and CAIX-TRuC
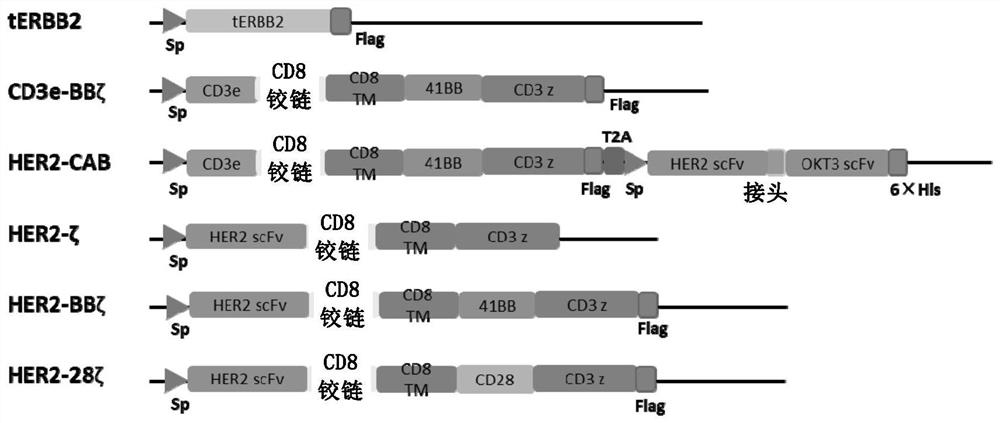
[0179] In order to verify the anti-tumor activity of CAB-T, in a set of experiments, we first designed the first set of structures using nanobodies targeting CAIX: CD3e-BBζ (amino acid sequence is SEQ ID NO.: 7), CAIX-BiTA ( The amino acid sequence is SEQ ID NO.:8), CAIX-CAB (amino acid sequence is SEQ ID NO.:9) and CAIX-TRuC (amino acid sequence is SEQ ID NO.:10). In order to distinguish the second group of CAIX-CAB structures described later, we named the CAIX-BiTA and CAIX-CAB of this group as 1 st -CAIX-BiTA and 1 st -CAIX-CAB. Among them, 1 st -CAIX-BiTA is BiTA without label, 1 st -CAIX-CAB is the CAB structure of CD3e-BBζ and untagged BiTA, and CAIX-TRuC is the TCR-utilized 2 The comparative structure of the company's platform technology. The specific structure diagram of the above structure is as ...
Embodiment 2
[0184] Example 2 Packaging of CAB and its control structure lentiviral vector
[0185] In the present invention, we use lentivirus as a vector to prepare CAB-T cells. First, we prepared lentiviral vectors carrying genes encoding CAB and its control constructs. The specific process of lentivirus packaging is as follows:
[0186] 1) Spread 1×10 in a 10cm culture plate 7 For HEK 293T cells, add 10 mL of DMEM (Hyclone, SH30243.01) medium containing 10% FBS (Gibco, 10099-141C), mix the cells well, and culture overnight at 37 degrees;
[0187] 2) On the second day, when the HEK 293T (ATCC, CRL-3216) cell confluence reaches about 90%, replace with serum-free DMEM;
[0188] 3) Prepare the plasmid complex, the amount of each plasmid is: 8 μg plasmad DNA, 4 μg psPAX2 and 2 μg pMD2g, dissolve in 1 mL opti-MEM (Gibco, 31985-070) and add 42 μL PEI (Polysciences, 24765-2); vortex Spin and shake for 20s. After standing at room temperature for 15 minutes, gently add the mixture to the HE...
Embodiment 3
[0192] Example 3 Preparation of CAB and its control structurally engineered T cells
[0193] After the lentiviral vector carrying the CAB structure is prepared, the lentiviral vector can be used to infect immune cells to complete the preparation of CAB-T cells. The specific procedure for the preparation of CAB-T cells is as follows:
[0194] 1) Culture commercial PBMC (Sai Li Bio, SLB-HP050B) cells with X-VIVO 15 (LONZA, 04-418Q) containing 5‰ human albumin (GRIFOLS, human albumin 20%), initially Cell density is 1 x 10 6 / mL;
[0195] 2) Add anti-CD3 / CD28beads (Miltenyi biotec, 130-091-441) according to the ratio of cells:Beads=3:1, and add 1000IU / mL IL-2 (Sihuan Biological, S10970016) to activate T cell expansion;
[0196] 3) After 48 hours of cell activation, an appropriate amount of virus and 12 μg / mL protamine (Sigma, P4005) were added to infect T cells;
[0197] 4) After 24 hours of lentivirus infection, the cell suspension was aspirated, and 1×10 6 Add complete f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com