Method for preparing anhydrous magnesium carbonate by directly utilizing salt lake brine
A technology of anhydrous magnesium carbonate and salt lake brine, applied in the direction of magnesium carbonate, etc., can solve the problems of good dispersibility of anhydrous magnesium carbonate, harsh reaction conditions, cumbersome preparation process, etc., to increase additional utilization value, simple equipment, and process flow simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
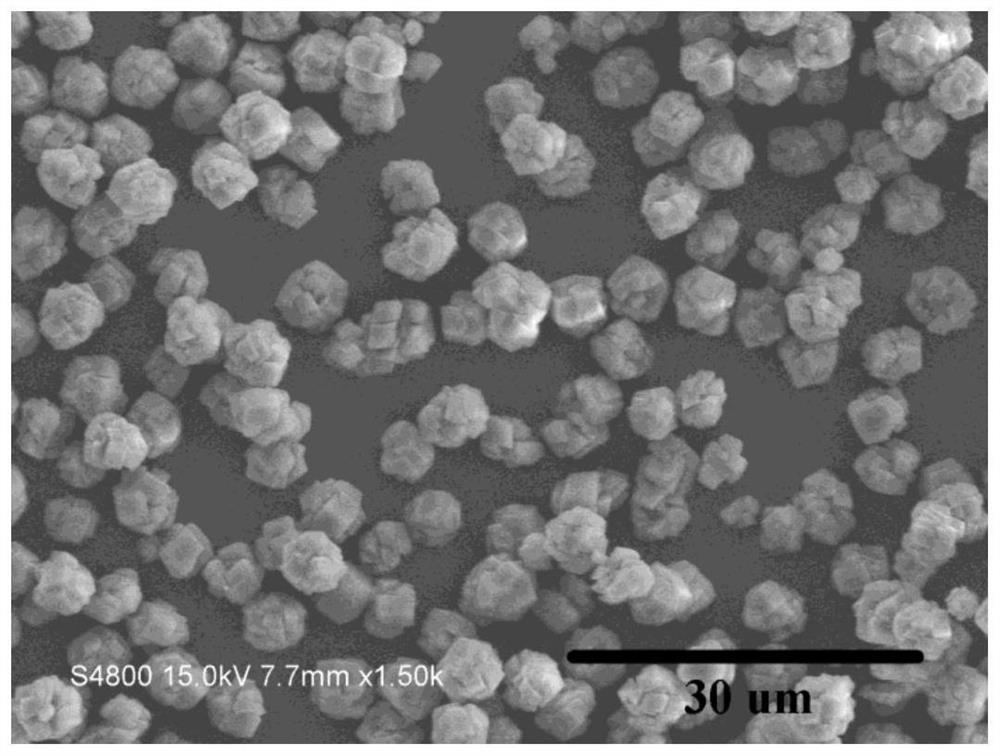
[0029] A kind of method that directly utilizes salt lake brine to prepare anhydrous magnesium carbonate, the steps of this method are as follows: add urea in brine, control urea and Mg in brine 2+ The molar ratio was 2.5:1, the initial pH of the mixed solution was adjusted to 8.5 with 5 mol / L NaOH solution, the above mixed solution was magnetically stirred for 20 min at a stirring speed of 200 r / min, and then placed in a 100 mL reaction kettle, Then put the reaction kettle into an oven, set the oven temperature to 180 °C, and cool the reaction kettle to room temperature after 6 hours of reaction to obtain the reaction solution; filter the reaction solution with suction, and then wash three times with deionized water until the pH value is 7.0 , and dried in an oven at 80 °C for 12 h to obtain anhydrous magnesium carbonate powder. Depend on figure 1 It can be seen that the particle size of the obtained anhydrous magnesium carbonate is about 5 um, and under this reaction system,...
Embodiment 2
[0031] A kind of method that directly utilizes salt lake brine to prepare anhydrous magnesium carbonate, the steps of this method are as follows: add urea in brine, control urea and Mg in brine 2+ The molar ratio was 2.0:1, the initial pH of the mixed solution was adjusted to 8.0 with 5 mol / L NaOH solution, the above mixed solution was magnetically stirred for 20 min at a stirring speed of 300 r / min, and then placed in a 100 mL reaction kettle, Then put the reaction kettle into an oven, set the oven temperature to 180 °C, and cool the reaction kettle to room temperature after 3 hours of reaction to obtain the reaction solution; filter the reaction solution with suction, and then wash with deionized water three times until the pH value is 7.0 , and dried in an oven at 80 °C for 12 h to obtain anhydrous magnesium carbonate powder. Depend on figure 2 It can be seen that the particle size of the obtained anhydrous magnesium carbonate is about 3~6 um, and under this reaction syst...
Embodiment 3
[0033] A kind of method that directly utilizes salt lake brine to prepare anhydrous magnesium carbonate, the steps of this method are as follows: add urea in brine, control urea and Mg in brine 2+ The molar ratio was 2.5:1, the initial pH of the mixed solution was adjusted to 9.0 with 5 mol / L NaOH solution, the above mixed solution was magnetically stirred for 20 min at a stirring speed of 400 r / min, and then placed in a 100 mL reaction kettle, Then put the reaction kettle into an oven, set the oven temperature to 180 °C, and cool the reaction kettle to room temperature after 2 hours of reaction to obtain the reaction solution; filter the reaction solution with suction, and then wash three times with deionized water until the pH value is 7.0 , and dried in an oven at 80 °C for 12 h to obtain anhydrous magnesium carbonate powder. Depend on image 3 It can be seen that the particle size of the obtained anhydrous magnesium carbonate is about 3~6 um, and under this reaction syste...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com