Aspirin and rivaroxaban compound preparation and preparation method thereof
A technology of rivaroxaban and aspirin, which is applied in the field of compound preparations of aspirin and rivaroxaban and its preparation, and can solve problems such as no cases of successful development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
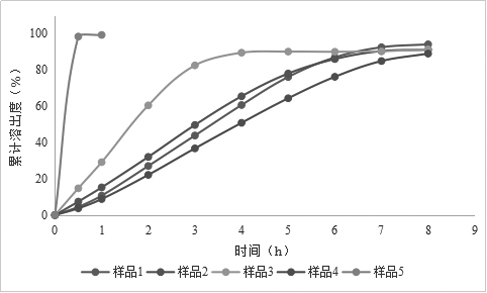
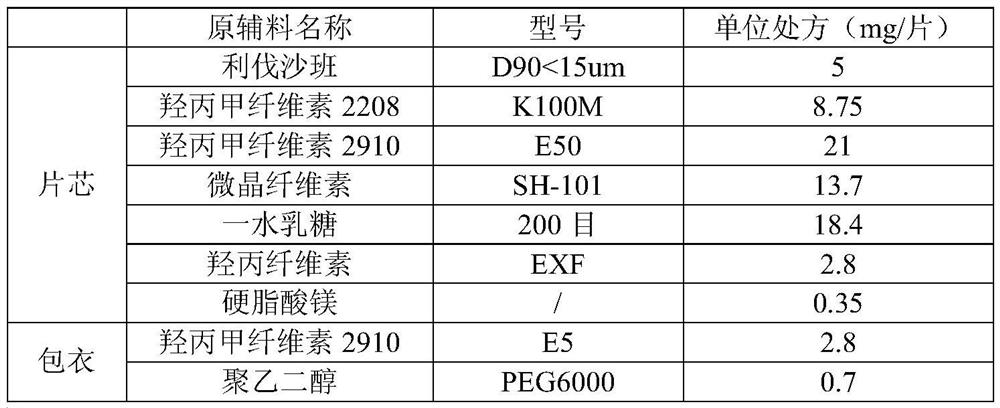
[0076] Example 1: Preparation of Rivaroxaban Sustained-release Tablets (I)
[0077] Table 1 Composition of Rivaroxaban Sustained-release Tablets (I)
[0078]
[0079] Preparation Process:
[0080] Granulation: add the other materials of the plain tablet except magnesium stearate into the wet granulator, the speed of the stirring paddle is 300rpm, the cutting knife is 500rpm, and mix for 5min; Finish adding in about 7 minutes; the speed of the stirring blade is 250rpm, the cutter is 1200rpm, and the granulation is 2min; the material is discharged, the screen of the granulator is 3×3mm, the speed is 400rpm, and the granules are wet granulated; the wet granules are added to the fluidized bed, and the air is 60°C , air volume 30Hz, drying, when the temperature of the material reaches 40°C, take a sample to measure the moisture, stop heating when the moisture is lower than 2.0%, cool and discharge; the screen diameter of the granulator is 1.0mm, the speed is 400rpm, dry granula...
Embodiment 2
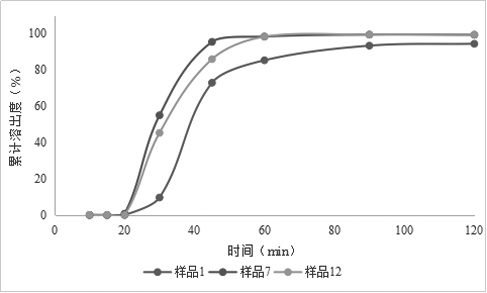
[0084] Example 2: Preparation of Rivaroxaban Sustained-release Tablets (II)
[0085] Table 2 Composition of Rivaroxaban Sustained-release Tablets (Ⅱ)
[0086] Name of raw material model Unit prescription (mg / tablet) Rivaroxaban D90<15um
5 Hypromellose 2280 K100M 6 Hypromellose 2910 E50 24 microcrystalline cellulose SH-101 25 lactose monohydrate 200 mesh 16 Hypromellose EXF 3.2 Magnesium stearate / 0.8 Hypromellose 2910 E5 3.2 polyethylene glycol PEG6000 0.8
[0087] Preparation Process:
[0088] Granulation: add the other materials of the plain tablet except magnesium stearate into the wet granulator, the speed of the stirring paddle is 300rpm, the cutting knife is 500rpm, and mix for 5min; Finish adding in about 7 minutes; the speed of the stirring blade is 250rpm, the cutter is 1200rpm, and the granulation is 2min; the material is discharged, the screen of the granulator is 3×3mm, the s...
Embodiment 3
[0092] Example 3: Preparation of Rivaroxaban Sustained-release Tablets (Ⅲ)
[0093] Table 3 Composition of Rivaroxaban Sustained-release Tablets (Ⅲ)
[0094] Name of raw material model Unit prescription (mg / tablet) Rivaroxaban D90<15um
1.25 Hypromellose 2280 K100M 2.5 Hypromellose 2910 E50 6 microcrystalline cellulose SH-101 4.1 lactose monohydrate 200 mesh 5.25 Hypromellose EXF 0.8 Magnesium stearate / 0.1 Hypromellose 2910 E5 1.6 polyethylene glycol PEG6000 0.4
[0095] Preparation Process:
[0096] Granulation: add the other materials of the plain tablet except magnesium stearate into the wet granulator, the speed of the stirring paddle is 300rpm, the cutting knife is 500rpm, and mix for 5min; Finish adding in about 7 minutes; the speed of the stirring blade is 250rpm, the cutter is 1200rpm, and the granulation is 2min; the material is discharged, the screen of the granulator is 3×3mm,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com