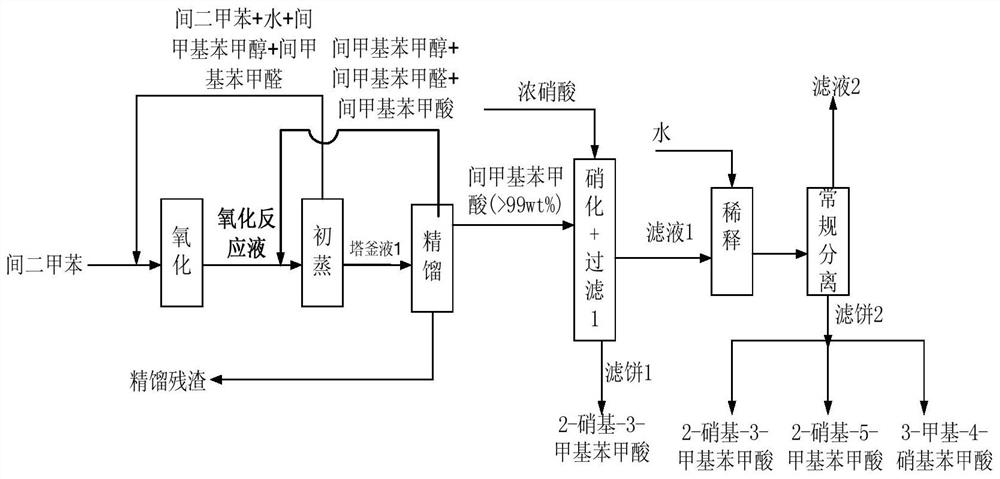
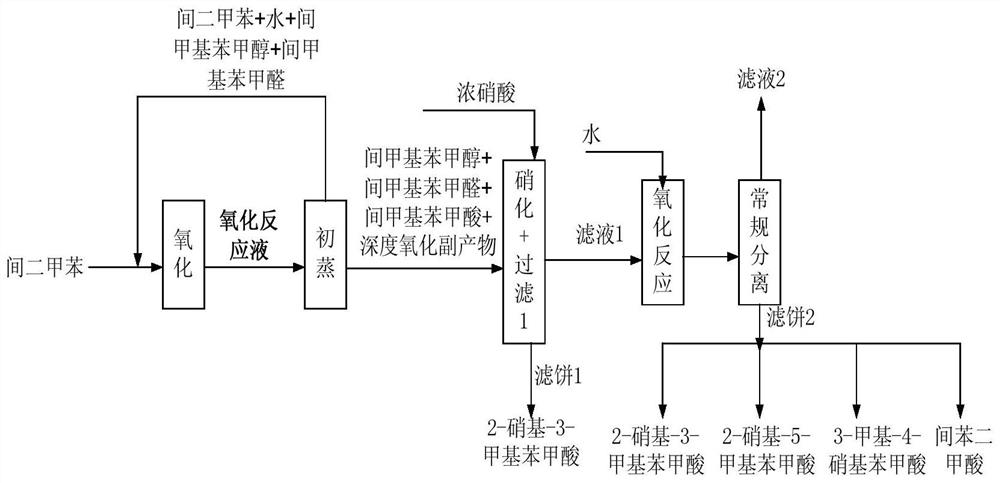
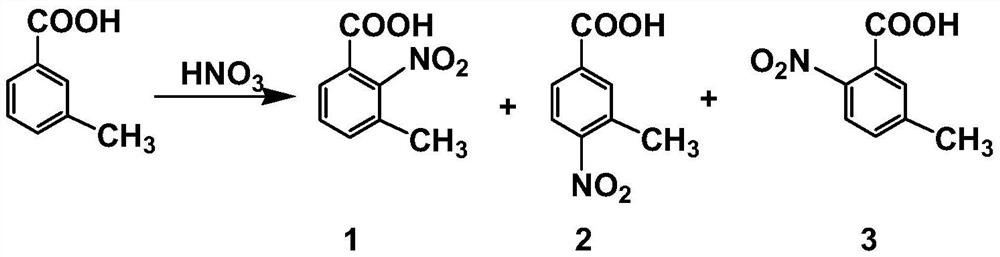
Method for co-producing m-toluic acid nitride and isophthalic acid
A technology of toluic acid and isophthalic acid, applied in chemical instruments and methods, preparation of nitro compounds, preparation of organic compounds, etc., can solve the problems of large amount of solid waste and low yield of target products, and achieve three wastes. The effect of reducing the amount of rectification, avoiding distillation residues, and improving the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080]Put 500 g of concentrated nitric acid with a mass fraction of 92% into a four-necked glass bottle with a volume of 1000 mL, start mechanical stirring, and cool down to -15°C. Slowly add 50 g of crude distillation tower liquid (0.3643 mol moles converted into xylene) into a glass bottle for reaction, keep stirring during the reaction and control the reaction temperature at -15°C, and stop the reaction after 10 minutes to obtain the main product It is the nitration reaction solution of 2-nitro-3-methylbenzoic acid. Through HPLC analysis, the conversion rate of raw materials m-toluic acid, m-methylbenzyl alcohol and m-methylbenzaldehyde is more than 99.5%, and the nitration reaction Liquid was filtered to obtain a filtrate and a filter cake. The filter cake was washed with water and dried to obtain 30.2 g of the product 2-nitro-3-methylbenzoic acid. The above-mentioned filtrate was mixed with water to form a 65wt% nitric acid aqueous solution, then the temperature was raise...
Embodiment 2
[0082] Put 500 g of concentrated nitric acid with a mass fraction of 94% into a four-neck glass bottle with a volume of 1000 mL, turn on mechanical stirring, and cool down to -17°C. Slowly add 66.6g of crude distillation tower liquid (0.4852mol moles converted into xylene) into a glass bottle for reaction, keep stirring during the reaction and control the reaction temperature at -17°C, and stop the reaction after 120 minutes to obtain the main The product is the nitration reaction solution of 2-nitro-3-methylbenzoic acid. Through HPLC analysis, the transformation rate of raw material m-toluic acid, m-methylbenzyl alcohol and m-tolualdehyde is all above 99.0%. The reaction solution was filtered to obtain a filtrate and a filter cake. The filter cake was washed with water and dried to obtain 41.3 g of the product 2-nitro-3-methylbenzoic acid. The above filtrate was mixed with water to form a nitric acid aqueous solution with a concentration of 45wt%, and then the temperature was...
Embodiment 3
[0084] Put 500 g of concentrated nitric acid with a mass fraction of 96% into a four-neck glass bottle with a volume of 1000 mL, turn on mechanical stirring, and cool down to -17.8°C. Slowly add 74.7g of crude distillation tower liquid (0.5443mol moles converted into xylene) into a glass bottle for reaction, keep stirring during the reaction and control the reaction temperature -17.8°C, and stop the reaction after 35 minutes to obtain the main The product is the nitration reaction solution of 2-nitro-3-methylbenzoic acid. Through HPLC analysis, the transformation rate of raw material m-toluic acid, m-methylbenzyl alcohol and m-tolualdehyde is all above 99.0%. The reaction solution was filtered to obtain a filtrate and a filter cake. The filter cake was washed with water and dried to obtain 47.1 g of the product 2-nitro-3-methylbenzoic acid. The above filtrate was mixed with water to form a 30wt% aqueous solution of nitric acid, then stirred and heated to 85° C., and reacted at...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com