In-situ carbonylation method straight-through liquid sodium phenylacetate synthesis method and application
A technology of sodium phenylacetate and a synthetic method, which is applied in chemical instruments and methods, preparation of organic compounds, separation/purification of carboxylic acid compounds, etc., can solve problems such as difficult industrial production, strong odor of phenylacetic acid, and adverse environmental effects. Achieve the effects of mild synthesis conditions, low price, and low waste water treatment cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
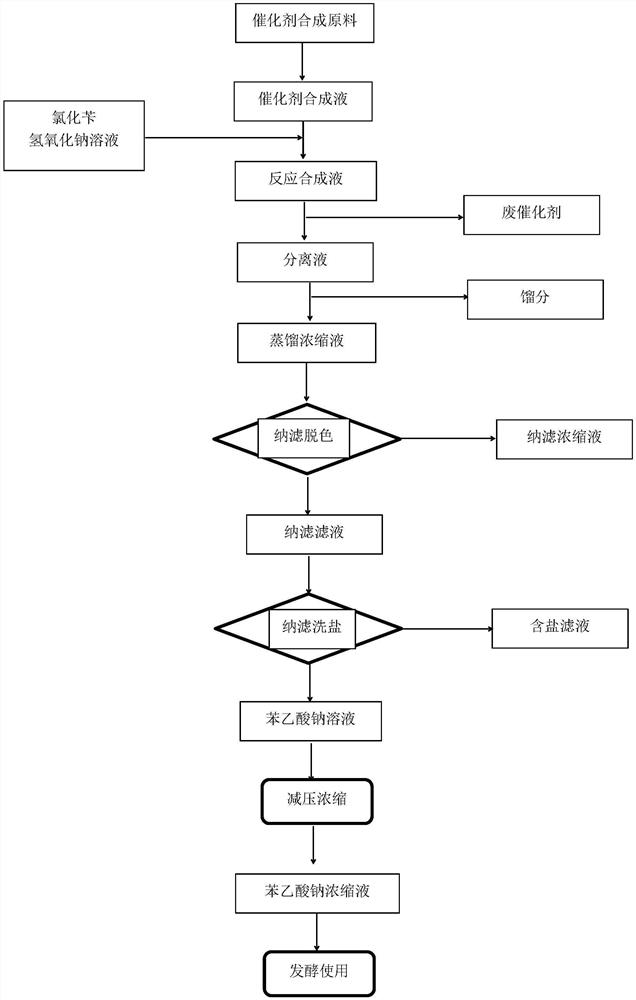
[0044] Such as figure 1 As shown, add industrial cobalt chloride (CoCl 2 ·6H 2 O) 476g, sodium thiosulfate (Na 2 S 2 o 3 ) 25g, metal manganese (200 mesh) 222g, sodium sulfide (Na 2 S) 48g, methanol 19.2L, and then use CO gas to replace 3 times, adjust the pressure to 0.08Mpa, control the reaction temperature to 35°C, adjust the speed to 800rad / min, and react for 5h.
[0045] In the reaction system of the synthetic catalyst, 24 kg of sodium hydroxide solution with a mass fraction of 40% and 15.2 kg of benzyl chloride were added dropwise, and the sodium hydroxide solution and benzyl chloride were added in about 3.5 hours (sodium hydroxide solution and chlorine Add benzyl chloride synchronously, without strictly controlling the pH during the process, then keep the temperature at 55°C and the pressure at 0.08MPa, and react for 6h.
[0046] After the reaction, the product in the reactor was aerated in situ for 2 hours; then filtered and separated, the filter cake was washed ...
Embodiment 2
[0056] The synthetic process of sodium phenylacetate is basically the same as in Example 1, the difference being that methanol and a small amount of organic phase are distilled out, and after the distilled concentrate obtained, nanofiltration decolorization and nanofiltration desalination are not carried out. The calculated yield is 95.9%, and the detected purity is 88.1%.
[0057] Carry out penicillin fermentation shake flask experiment according to embodiment 1 method:
[0058] The potency of the sodium phenylacetate shake flask prepared in this example is 15600 U / mL, which is more than 5% different from the result of the control group in Example 1, which does not meet the requirements of the fermentation experiment. It can be seen that the direct synthesis of sodium phenylacetate used for penicillin fermentation must be decolorized by nanofiltration and desalted by nanofiltration.
Embodiment 3
[0060] The synthesis process of sodium phenylacetate is the same as in Example 1, the difference is that the concentration of sodium phenylacetate is directly detected without using a nanofiltration membrane to desalinate after decolorization, and the calculated yield is 94.4%, and the detected purity is 98.1%.
[0061] Carry out penicillin fermentation shake flask experiment according to embodiment 1 method:
[0062] The potency of the sodium phenylacetate shake flask prepared in this example is 18000U / mL, which is more than 5% different from the result of the control group in Example 1, which does not meet the requirements of the fermentation experiment. It can be seen that the sodium phenylacetate used for direct synthesis of penicillin fermentation must be desalted by nanofiltration.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com