Method for determining concentrations of valsartan and sacubitril in human plasma and application of valsartan and sacubitril
A sacubitril and determination method technology, applied in the directions of measuring devices, instruments, scientific instruments, etc., can solve the problem that valsartan and sacubitril are not suitable for clinical pharmacokinetic detection, and achieve good reproducibility, The effect of avoiding dilution and reducing workload
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
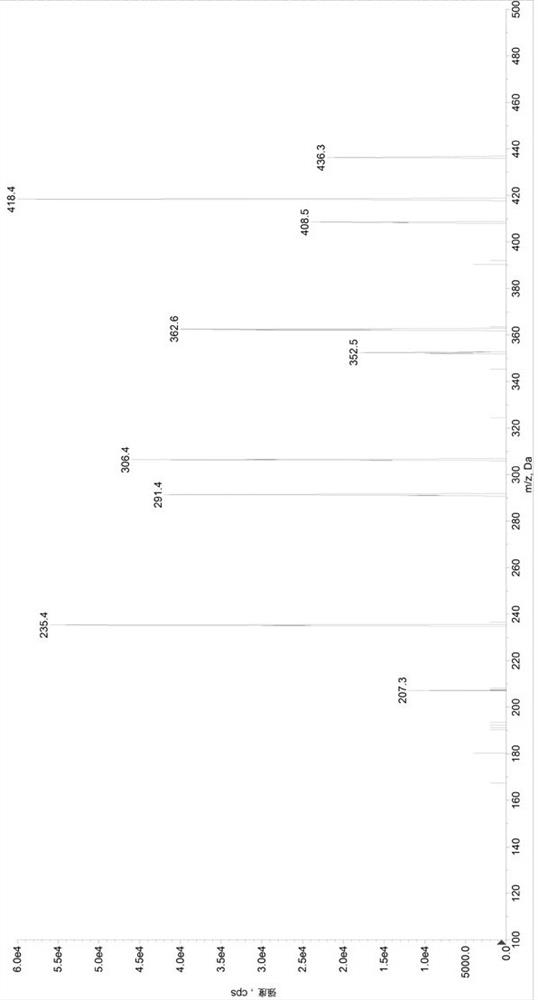
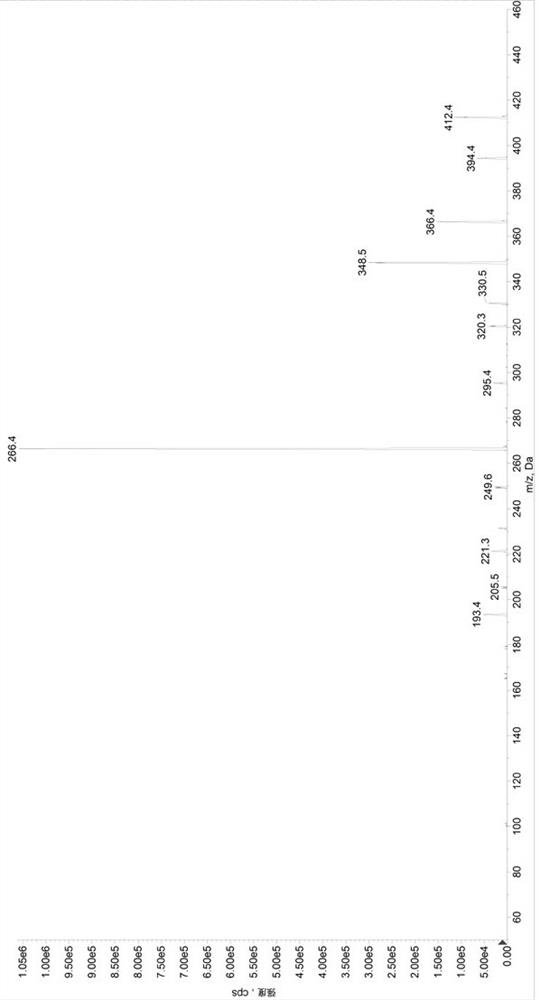
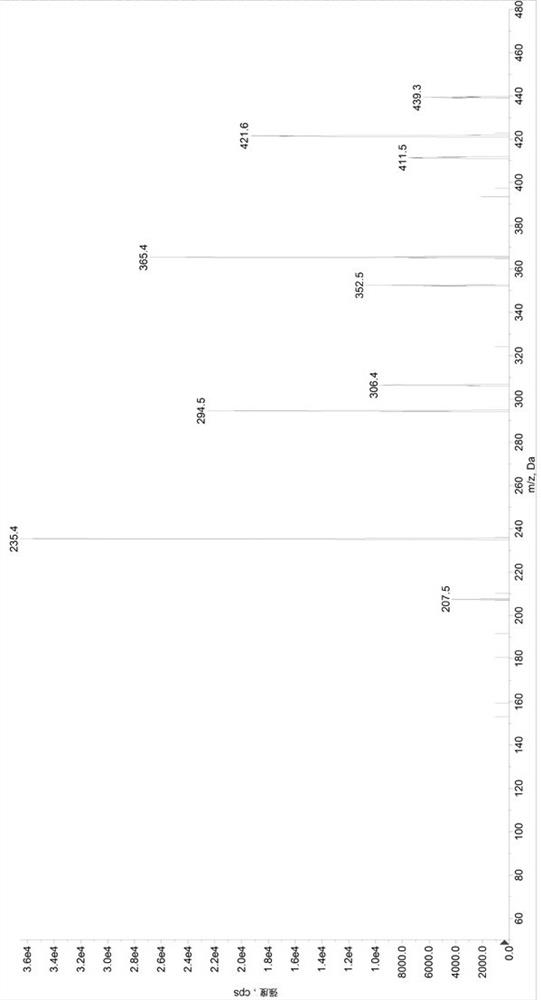
[0097] Preparation of control group (SET1) samples: Add 380µL of diluent 2 (methanol / water, 1 / 1, volume ratio) to a 1.5mL centrifuge tube, add 20µL of quality control mixed working solution (low concentration quality control working solution / medium concentration quality control working solution / high concentration quality control working solution) to obtain sample solutions with valsartan and sacubitril concentrations of 22.5 / 15ng / mL, 600 / 400ng / mL, and 5700 / 3800ng / mL, respectively, in a 96-well plate Add 50µL sample solution and 50µL internal standard solution, add 500µL 30% acetonitrile aqueous solution precisely, vortex mix, centrifuge at 6000g for 10min, draw 100µL supernatant into a new 96-well plate, add 200µL 30% acetonitrile water to dilute , after vortex mixing, centrifuge at 6000g, 5°C for 5min, and take 5µL for HPLC-MS / MS analysis. Prepare 3 samples for each concentration, record the chromatogram, record the peak area of valsartan (A), calculate its average value As...
Embodiment
[0156] Example: Human Bioequivalence Study of Sacubitril-Valsartan Sodium Tablets
[0157] Taking Chinese healthy volunteers as subjects, evaluate sacubitril valsartan calcium sodium tablets (100mg: 1 tablet, test preparation) developed and produced by a certain company orally on an empty stomach and sacubitril valsartan produced by Novartis The relative bioavailability of sodium tablets, trade name Nuoxintuo® (100mg: 1 tablet, reference preparation), evaluates whether the test preparation and the reference preparation are or may be bioequivalent. This study is a single-center, randomized, open-label, two-drug, single-administration, three-cycle partially repeated crossover, fasting, and postprandial bioequivalence experiment in healthy subjects.
[0158] Design blood collection points: 0 hours after administration (within 60 minutes before administration) and 0.167 (10min), 0.333 (20min), 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 3.5, 4, 5 after administration , 6, 8, 12, 24, 36, 48, an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com