Biocatalytic preparation method of tauroursodeoxycholic acid
A technology of tauroursodeoxycholic acid and biocatalysis, which is applied in chemical instruments and methods, preparation of hydroxyl compounds, preparation of organic compounds, etc., can solve the problem of economy and stability, which is difficult to achieve industrial production, and does not have the conditions for large-scale production , Difficulty in post-extraction, etc., to achieve the effects of easy recycling, reduction of subsequent processing procedures, and cost optimization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
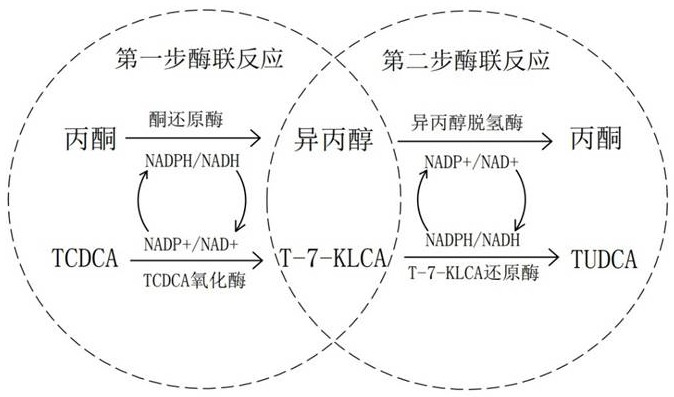
[0024] Example 1: Reaction system 500ml, first add 125ml of acetone and 300ml of pure water into a 1L three-hole reaction flask to stir and mix, put the reaction flask in a constant temperature water bath at 25°C, then add 50.16g of 98% pure TCDCA to continue stirring For half an hour, let it fully dissolve into the mixed solution, then add 0.05g NADP+, adjust the pH to 6.0~6.2, then add 5.35g ketoreductase (immobilized enzyme) and 10.52g TCDCA oxidase (immobilized enzyme), use Make up the volume with pure water to 500ml, react at 150rpm for 15~20h, control the temperature at 25~26℃, pH6.0~6.2 during the reaction, and detect the content of TCDCA and T-7-KLCA with liquid phase, when the substrate reaction exceeds 99.0% Terminate the reaction, pass the reaction solution through a 60-mesh filter to recover the immobilized enzyme, and the rest of the system waits for the second step of enzyme reaction.
Embodiment 2
[0025] Example 2: The reaction system is about 600ml. First, add the reaction solution after removing the enzyme in Example 1 into a 1L three-hole reaction bottle, place the reaction bottle in a constant temperature water bath at 25°C, turn on the stirring at 150rpm, and add 75ml to the reaction solution Isopropanol, adjust the pH to 6.0-6.2, then add 10.13g of isopropanol dehydrogenase enzyme solution and 15.62g of T-7-KLCA reductase enzyme solution, react at 150rpm for 15-20h, control the temperature and pH during the reaction Within the required range, and detect the content of T-7-KLCA and TUDCA with liquid phase, stop the reaction when the substrate reaction exceeds 99.0%, adjust the pH of the reaction solution to 4.0 with concentrated hydrochloric acid, continue stirring for 3 hours, and centrifuge at 10,000g for 10 minutes to remove the precipitate After the supernatant was collected, it was filtered with a 0.1 μm microporous membrane, and the remaining reaction solution...
Embodiment 3
[0026] Example 3: The reaction system is 1000ml. First, add 375ml of acetone and 500ml of pure water into a 2L three-hole reaction flask to stir and mix. The reaction flask is placed in a constant temperature water bath at 37°C, and then 250.11g of TCDCA with a purity of 98% is added to continue stirring Half an hour, let it fully dissolve into the mixed solution, then add 0.20g NAD+, adjust the pH to 8.3~8.5, then add 75.59g ketoreductase (immobilized enzyme) and 124.64g TCDCA oxidase enzyme solution (immobilized enzyme) , use pure water to make up the volume to 1000ml, react at 300rpm for 10~15h, control the temperature during the reaction at 36~37℃, pH8.3~8.5, and use the liquid phase to detect the content of TCDCA and T-7-KLCA, when the substrate reaction exceeds 99.0 %, the reaction was terminated, the reaction solution passed through a 60-mesh filter to recover the immobilized enzyme, and the rest of the system waited to enter the second step of the enzyme reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com