Method for detecting in-vivo psychotropic drugs and metabolites thereof, and application thereof
A technology for metabolites and drugs, applied in the field of blood drug concentration detection, can solve the problems of increasing analytical throughput, sensitivity, chromatographic peak capacity, and expensive equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: complete detection method and standard solution sample injection
[0042] A method for detecting psychotropic drugs and metabolites thereof in blood or hair, said method adopting ultra-high performance liquid chromatography-ultraviolet detection method (i.e. UPLC-UV method), comprising the following steps:
[0043] S1 chromatographic conditions: Chromatographic column: C18 chromatographic column, ACQUITY UPLC BEH C18 diameter 2.1mm*length 50mm, its filler particle size is 1.7μm; mobile phase: A phase is 0.05% v / v formic acid-water solution, B phase is 0.05% v / v formic acid-methanol solution; detector is ultraviolet (UV) DAD detector, UVλ max : Full wavelength scanning; Column temperature: 40°C; Mobile phase flow rate: 0.2mL / min; Injection volume: 10.0μL;
[0044] The preparation of S2 working solution includes:
[0045] S20: Preparation of needle washing solution: Measure 700mL of water into the liquid phase washing bottle with a graduated cylinder, then...
Embodiment 2
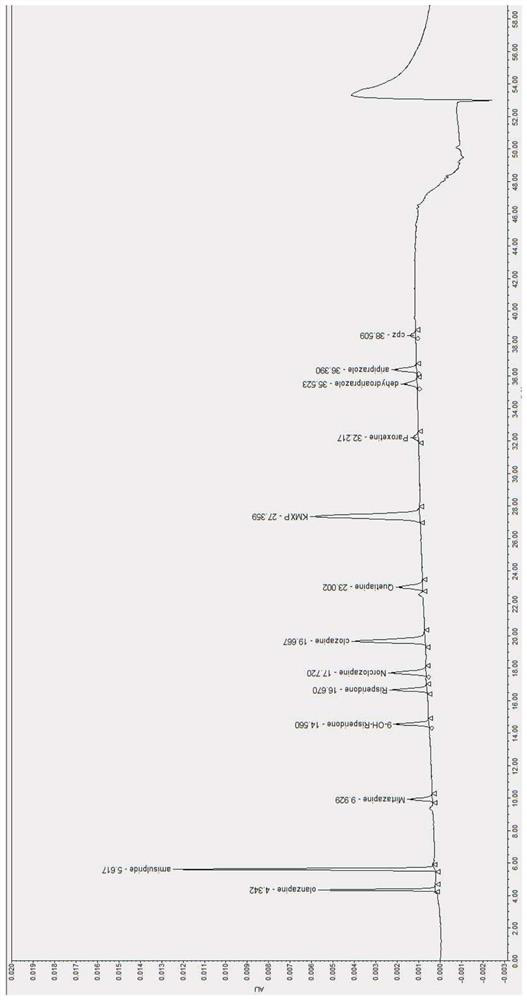
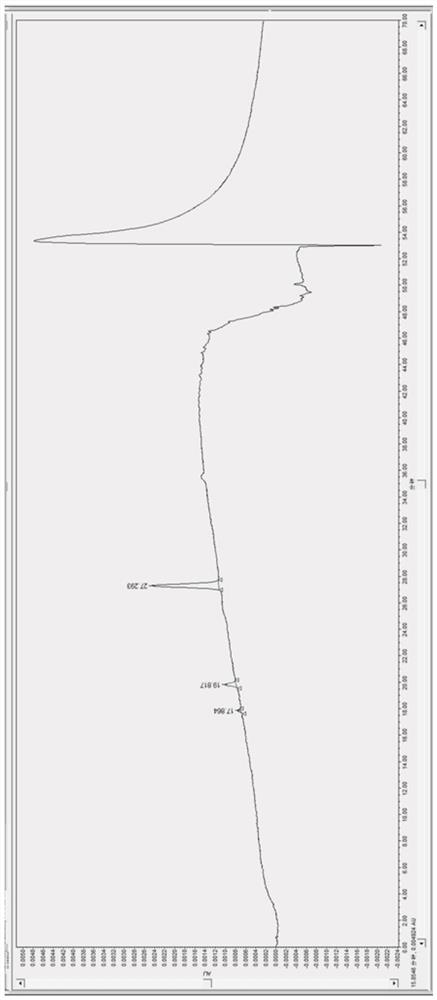
[0051] Embodiment 2 sample serum: patient's serum chromatogram of taking clozapine medicine
[0052] The preparation method of S1 chromatographic condition, S21 internal standard solution, S22 mixed standard solution is the same as embodiment 1;
[0053] Preparation of S231 serum test solution: S2311: Take 20 μL of the internal standard solution prepared in the above step S21 and dry it with nitrogen gas, mix it with 500 μL of thawed frozen serum sample to be tested into a 7 mL centrifuge tube, vortex for 1 min for the first time, and add 200 μL Aqueous sodium hydroxide solution with a concentration of 2mol / L, then add 3mL of methyl tert-butyl ether, and then vortex for 3min for the second time; The final supernatant was blown dry with nitrogen gas, then redissolved in 80 μL 50% v / v methanol aqueous solution, vortexed for 1 min, and the product was obtained;
[0054] S3 assay method: need testing solution is injected ultra-high performance liquid chromatograph, adopts gradien...
Embodiment 3
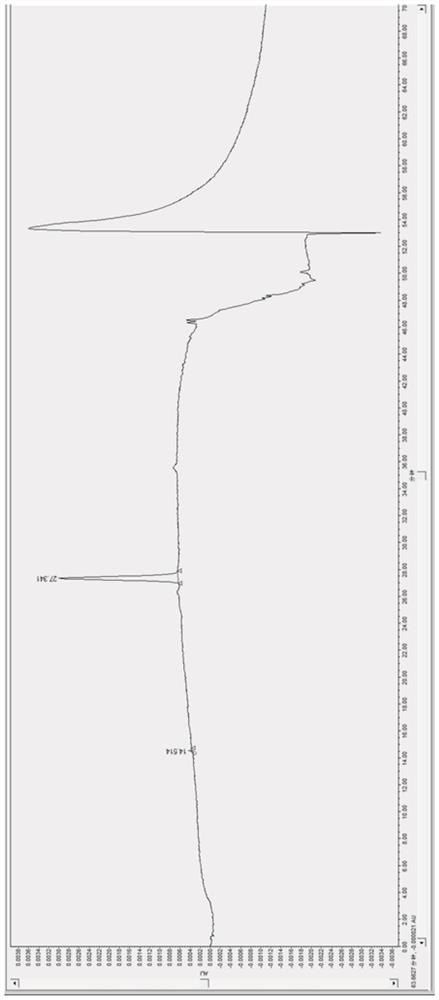
[0055] Embodiment 3 sample serum: the serum chromatogram of the patient who takes paliperidone (9-OH risperidone)
[0056] The preparation method of S1 chromatographic condition, S21 internal standard solution, S22 mixed standard solution is the same as embodiment 1;
[0057] S231 need testing solution preparation, S3 measuring method are with embodiment 2: as image 3 As shown, obtain the retention time of the psychotropic drug and its metabolites (t R ), that is; the retention time t of paliperidone R 14.514min, carbamazepine 27.341min (internal standard).
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com