A kind of N-aryl-substituted carbazole fluorescent probe capable of specifically labeling cell membrane and preparation method thereof
A fluorescent probe and cell membrane technology, applied in the field of fluorescent probes, can solve the problems of poor specificity and low contrast of fluorescence imaging, and achieve the effects of reducing self-absorption, realizing fluorescence imaging, and achieving contrast
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
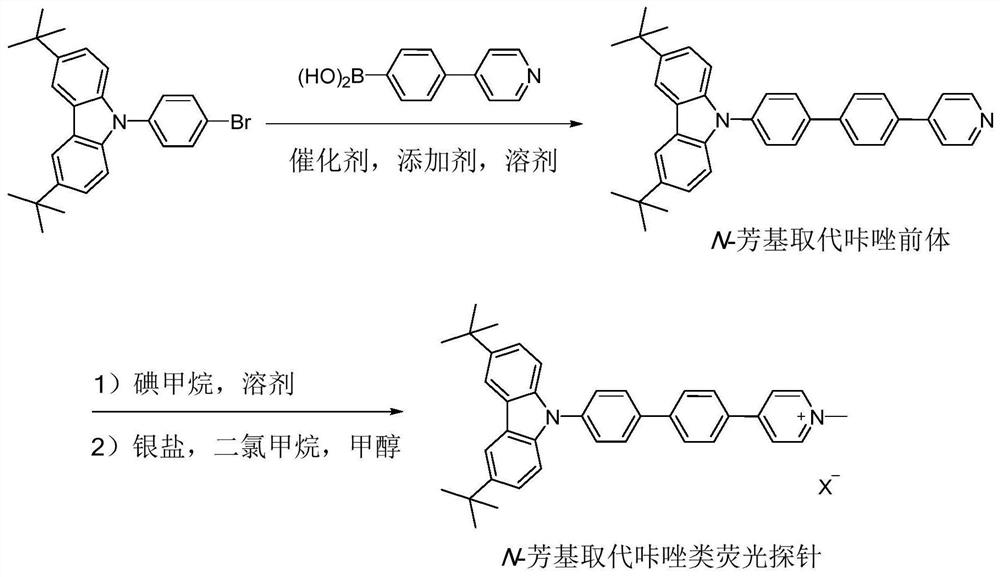
[0030] The synthesis of N-aryl substituted carbazole fluorescent probe CZDPPy capable of specifically labeling cell membranes comprises the following steps:
[0031] Step (1): Add 9-(4-bromophenyl)-3,6-di-tert-butylcarbazole (1.04g, 2.4mmol), 4-(4-pyridyl)phenylboronic acid to the dry reactor (398mg, 2.0mmol), Pd(PPh 3 ) 4 (116 mg, 0.1 mmol), Na 2 CO 3 (636mg, 6.0mmol), toluene (20mL), ethanol (6mL), water (2mL), react at 110°C for 24 hours under nitrogen protection. Cool to room temperature, add 20mL ethyl acetate to dilute, wash with saturated sodium chloride solution, anhydrous Na 2 SO 4 dry. After collecting the organic phase, the solvent was removed under reduced pressure, and the residue was separated and purified by silica gel column chromatography (petroleum ether / ethyl acetate=2 / 1, v / v), and vacuum-dried to obtain the N-aryl substituted carbazole precursor ( 814 mg, yield 80%);
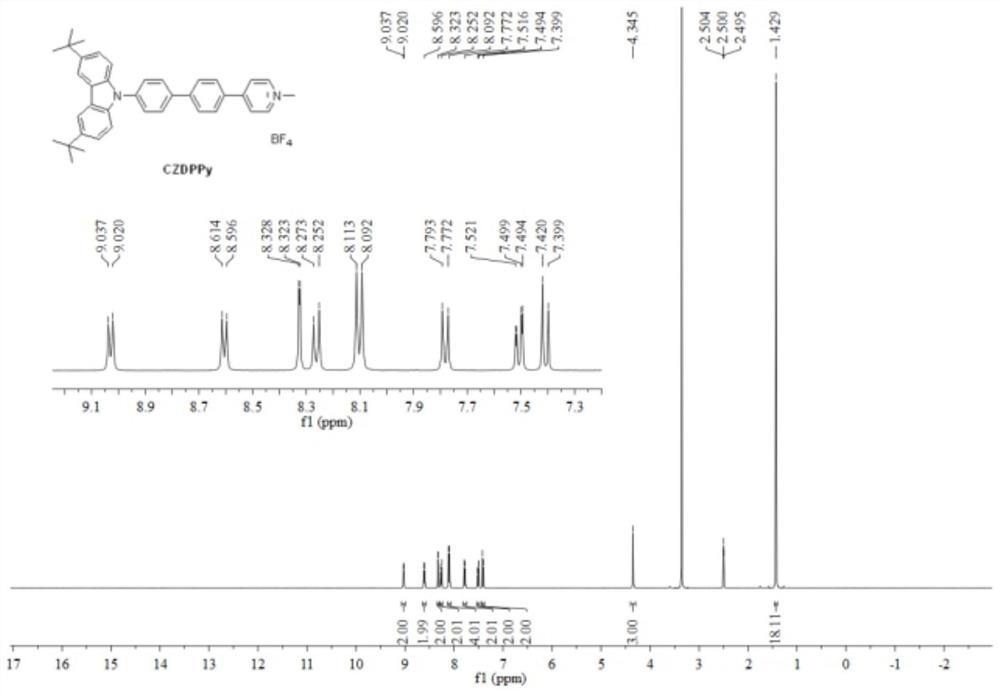
[0032] Step (2): Add N-aryl-substituted carbazole precursor (200 mg, 0.4 mmol), m...
experiment example 1
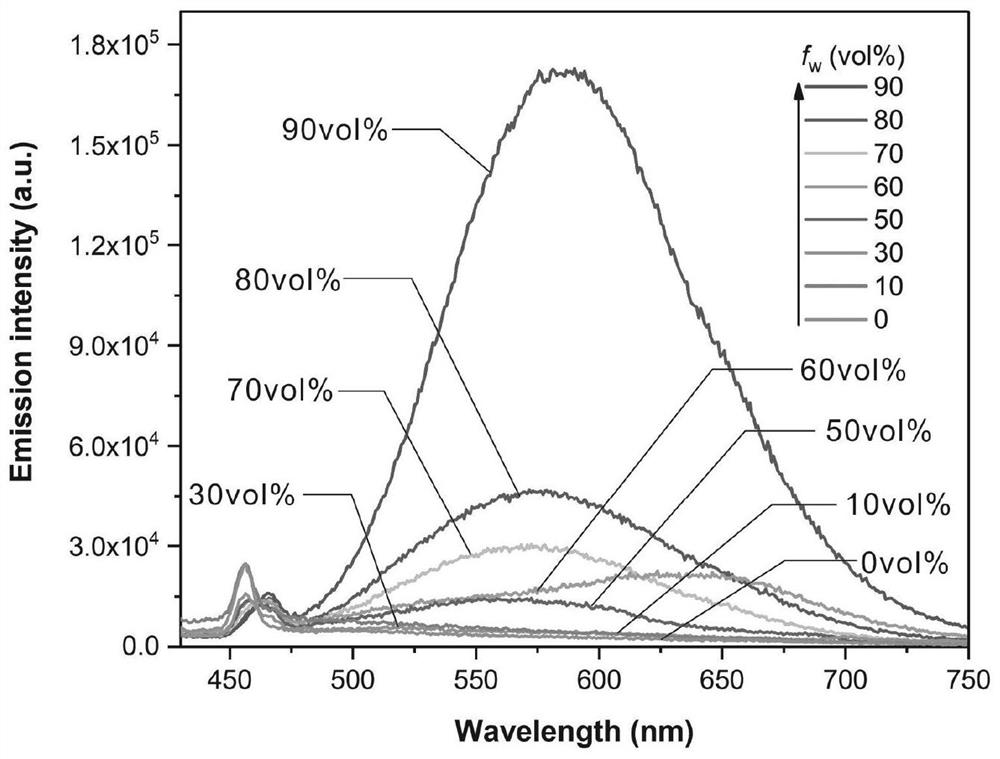
[0041] Experimental example 1: fluorescent probe CZDPPy in DMSO / H 2 Aggregation-induced Luminescence in O System
[0042] Prepare DMSO / H with a concentration of 10 μM and water volume percentages of 10%, 30%, 50%, 60%, 70%, 80% and 90% of CZDPPy 2 O solution, then test the fluorescence emission spectrum of each solution and compare the change of fluorescence intensity at the maximum emission wavelength, the test results are as follows: image 3 shown. Among them, the instruments used for spectral characterization are: HITACHI U-2910 ultraviolet-visible spectrophotometer (scanning range 250-1100 nm), Horiba Fluoromax-4 fluorescence spectrometer.
[0043] Depend on image 3 It can be seen that the fluorescent probe CZDPPy has a strong intramolecular charge transfer effect and a large Stokes shift. 2 In the mixed solvent of O, the maximum fluorescence emission wavelength reaches the red light region of 580nm, which effectively reduces the self-absorption of fluorescent dyes a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com