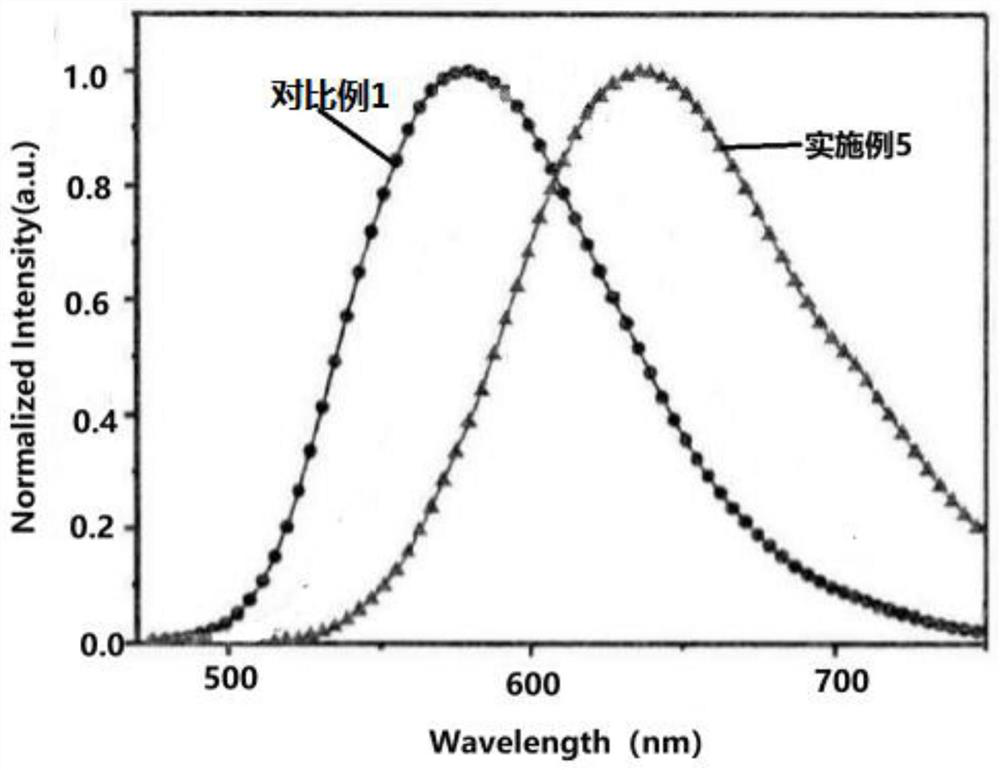
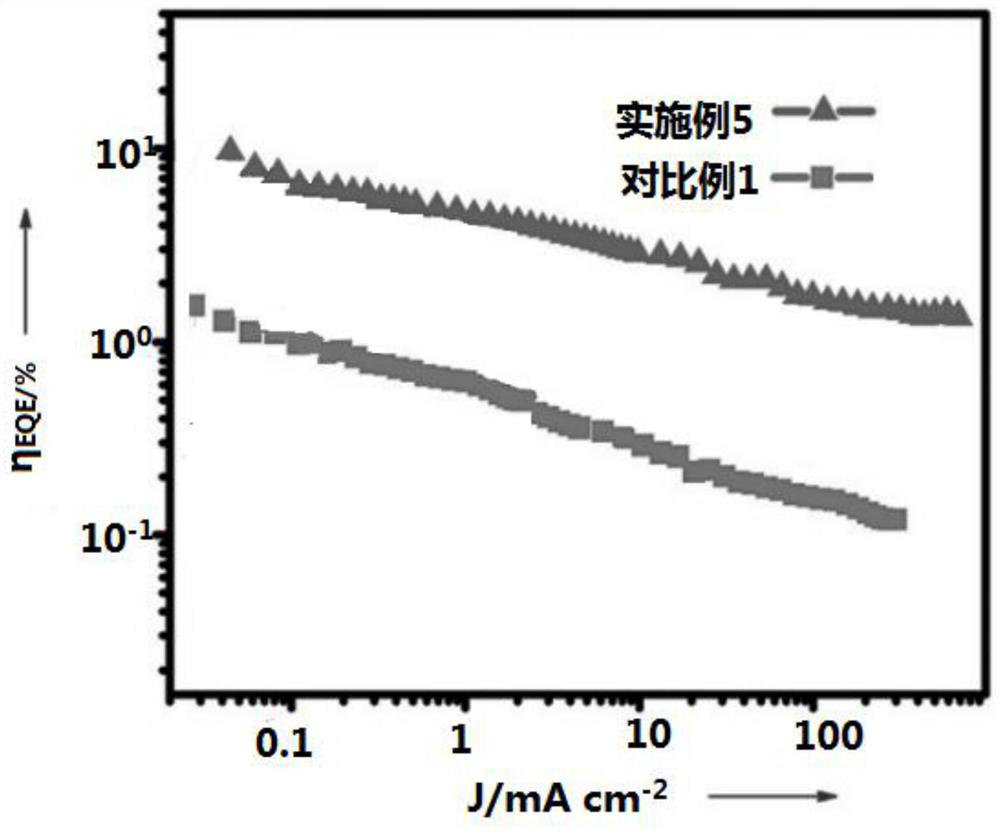
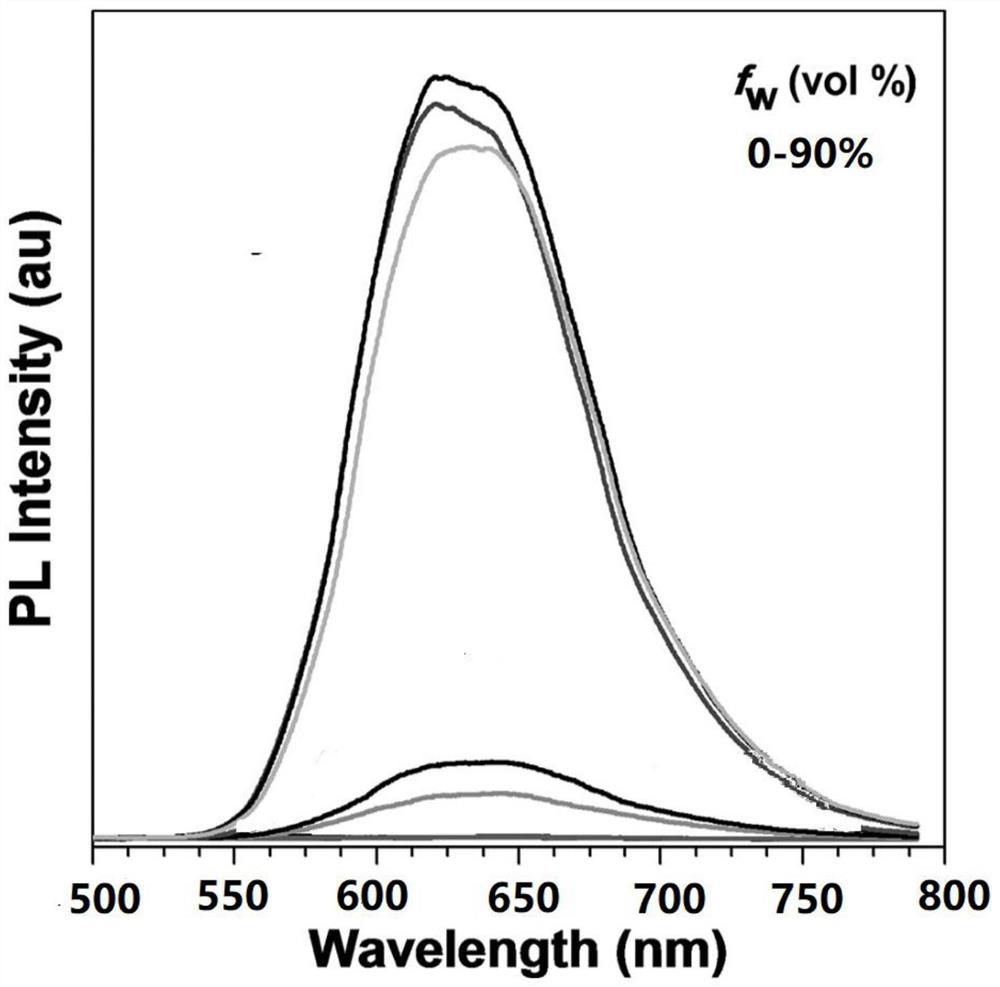
Multifunctional delayed fluorescence material and preparation method thereof
A delayed fluorescence and multifunctional technology, which is applied in the fields of luminescent materials, chemical instruments and methods, semiconductor/solid-state device manufacturing, etc., can solve the problem of low efficiency of TADF small molecule red light materials, and achieve the improvement of external quantum efficiency and luminous intensity , Improve the effect of charge transfer effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] At room temperature, the compound (2.69g, 10mmol) shown in the formula (V) was added in a 100ml single-port reaction flask, and then an appropriate amount of solvent THF was added until the compound shown in the formula (V) was completely dissolved (about 80ml), and the liquid bromine ( 2ml, 40mmol) was dissolved in 3ml of THF solution, added dropwise to the reaction system, and reacted in the dark for 8 hours. Add water to stop the reaction, extract with DCM, wash several times with a large amount of deionized water, collect the organic liquid, and concentrate. The crude product is separated and purified by column chromatography to obtain compound (2.089g, yield 60%) shown in formula (IV), and its chemical reaction equation is:
[0023]
Embodiment 2
[0025] With 4,4,5,5-tetramethyl-2-(pyran-1-yl)-1,3,2-dioxaborane (4.92g, 15mmol), compound shown in formula (IV) ( 3.48g, 10mmol) and catalyst Pd (PPh 3 ) 4 (0.62 g, 0.5 mmol) were mixed in toluene (80 mL). Will K 2 CO 3 Aqueous solution (2.03 g, 15 mmol) was slowly added to the reaction mixture, and after bubbling nitrogen for 30 min, the reaction mixture was heated to 85° C. under nitrogen and refluxed for 12 h (reaction progress was monitored by TLC). After the reaction was complete, it was diluted with ethyl acetate (150 mL). The organic layer was washed with brine solution, water and washed with anhydrous Na 2 SO 4 Dry, filter, and remove solvent in vacuo, obtain compound (3.18g, productive rate 68%) shown in product formula (III), its chemical reaction equation is:
[0026]
Embodiment 3
[0028] Add the compound represented by formula (III) (11.7g, 25mmol), glacial acetic acid (25ml) and concentrated sulfuric acid (75ml) into a 500ml single-necked bottle at 0-5°C, and stir in the dark. Then N-bromosuccinimide (NBS) (13.9 g, 80 mmol) was added three times, and then gradually raised to room temperature, and reacted overnight. The reaction solution was diluted with a large amount of water, the solid was separated, and then repeatedly washed with NaHCO 3 Aqueous solution, methanol wash several times, after drying, purify with hot chlorobenzene solvent, obtain the compound (13.76g, productive rate 75%) shown in formula (II), its chemical reaction equation is:
[0029]
PUM
| Property | Measurement | Unit |
|---|---|---|
| emission peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com