Co-based catalyst for breaking restrictive relationship of synthetic ammonia reaction and preparation method and application thereof
A catalyst and a technology for synthesizing ammonia, applied in the direction of ammonia preparation/separation, chemical instruments and methods, physical/chemical process catalysts, etc., can solve problems that have not been reported yet, reduce the energy barrier of catalytic reactions, increase service life, and improve reaction speed effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
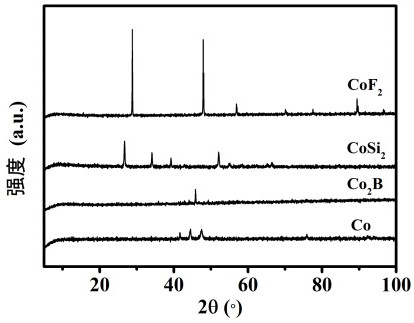
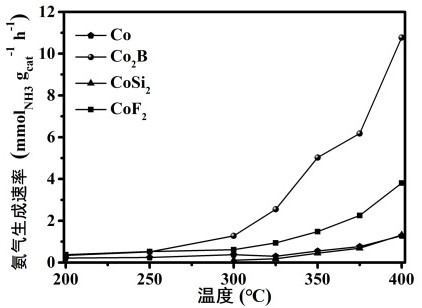
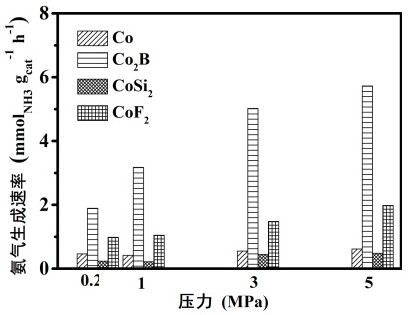
Image
Examples
Embodiment 1
[0023] co 2 Preparation of B
[0024] Cobalt acetate (0.498 g, 0.1 M) was dissolved in deionized aqueous solution mixed with 50% methanol, and then sodium borohydride solution (0.228 g, 1 M) was added dropwise to the above solution under nitrogen flow, and after the addition was completed, Continue to react for 1 h. The subsequently formed black product was thoroughly washed three times with deionized water and three times with anhydrous methanol. get co 2 B-based catalyst.
Embodiment 2
[0026] CoF 2 preparation of
[0027] Cobalt nitrate (0.29 g), ammonium fluoride (0.12 g), and urea (0.3 g) were dissolved in 50 mL of ethylene glycol, and a homogeneous solution was formed under vigorous stirring. Then, the obtained solution was transferred into a polytetrafluoroethylene hydrothermal kettle and placed in an oven at 200 °C for 20 h. After cooling to room temperature, the pink product was collected and washed three times with water and ethanol, respectively. Get CoF 2 base catalyst.
Embodiment 3
[0029] co 2 Preparation of Si
[0030] Silicon powder (0.29 g), cobalt tetraoxide powder (0.80 g) and magnesium powder (2.40 g) were mixed and put into a 20 mL stainless steel medium-high pressure reactor. Then the high reactor was sealed and heated at 10 ℃·min -1 The heating rate was from room temperature to 750 °C and kept for 10 h. After the autoclave was naturally cooled to room temperature, the obtained precipitate was washed three times with ethanol, distilled water, and hydrochloric acid solution (0.50 M). Finally dried in vacuum at 50 °C overnight to obtain Co 2 Si-based catalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com