Pseudosciaena crocea antitumor peptide piscidin 5 like and preparation method and application thereof
A technology of rlc-p5l and pet-28a, which is applied in the fields of antineoplastic drugs, botanical equipment and methods, biochemical equipment and methods, etc., can solve the problems of tumor or inflammation treatment failure, and achieve low production cost and simple expression system , strong biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
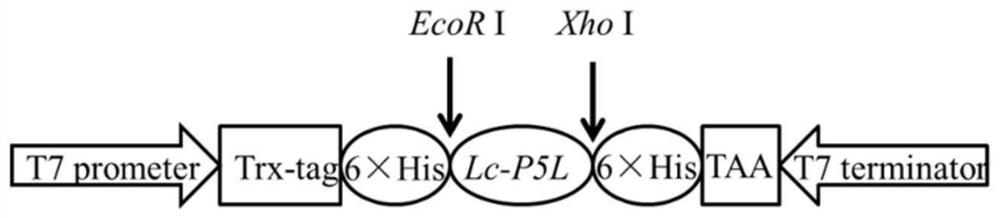
[0052] The construction of embodiment 1 large yellow croaker Lc-P5L prokaryotic recombinant expression vector
[0053] According to the multiple cloning site of the pET-28a vector, specific primers F1 / R1 with restriction endonuclease sites were designed to amplify the sequence encoding the signal peptide in the ORF of the piscidin 5 like gene of large yellow croaker. An EcoR I restriction site was added to the 5' end of the forward primer F1; an Xho I restriction site was added to the 5' end of the downstream primer R1.
[0054] Upstream primer F1: 5′-CCG GAATTC GGAGACAACTACGGTACTTTC-3';
[0055] Downstream primer R1: 5′-CCG CTCGAG TTTGCTGCCGTCGTCCT-3'.
[0056] A fragment of the coding region of Lc-P5L was amplified. The PCR reaction conditions are: pre-denaturation at 94°C for 5 minutes; denaturation at 94°C for 45 seconds, and denaturation at 58°C
[0057] Anneal for 45s, extend for 45s at 72°C, repeat 35 cycles; extend for 10min at 72°C.
[0058] The PCR product wa...
Embodiment 2
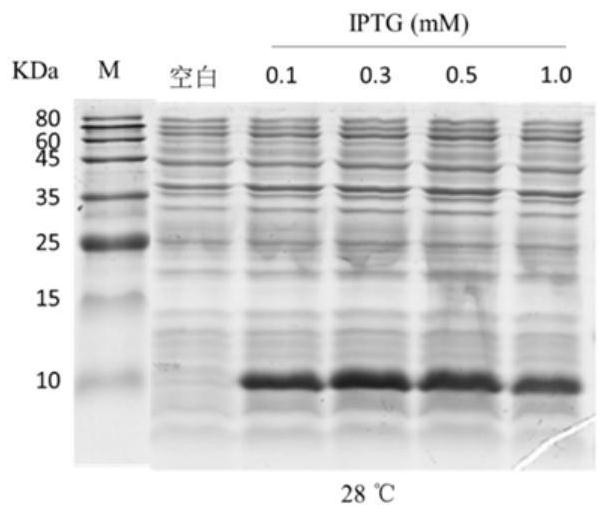
[0060] Example 2 Induced expression of pET-28a-Lc-P5L recombinant plasmid in E.coli BL21(DE3): The correctly sequenced plasmid pET-28a-Lc-P5L was transformed into E.coli BL21(DE3) large intestine by heat shock method Bacillus and induce expression with IPTG.
[0061] The results show that, compared with before induction, E.coliBL21 (DE3) transformed with pET-28a-Lc-P5L recombinant plasmid has obvious induced expression of recombinant protein, and the protein band is around 10KDa (see figure 2 ).
Embodiment 3
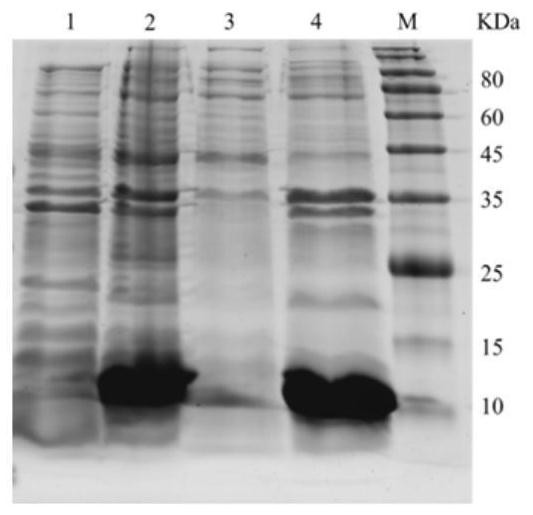
[0062] Example 3 Purification of expression products after IPTG induction in E.coli BL21(DE3) transformed with pET-28a-Lc-P5L recombinant plasmid
[0063] The rLc-P5L recombinant protein was purified by affinity chromatography. After a large amount of positive recombinant E.coliBL21 (DE3) was induced and expressed, it was centrifuged at 12000rpm at 4°C for 10min to remove the supernatant, and an appropriate amount of sonication solution (50mM Tris-HCl, 0.5M NaCl, 1 mM EDTA, pH 8.0), after crushing by high-pressure ultrasound, centrifuge at 12000 rpm at 4°C for 30 min to collect the precipitate. Add an appropriate amount of inclusion body washing solution (50mM Tris-HCl, 0.5M NaCl, 2M urea, 1% Triton X-100, pH7.8) to resuspend the bacteria, wash the inclusion body 3 times, add inclusion body lysis solution (100mM Triton -HCl, 500mM Nacl, 8M urea, 20mM imidazole, pH 7.4; 0.3mg / ml and 2% Triton-X 100 (added when used)), the supernatant was filtered through a 0.22μm filter membran...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com