Lenvatinib medicine composition and preparation method thereof
A technology of lenvatinib and its composition, which is applied in the field of pharmaceutical composition containing lenvatinib or its salt and its preparation, can solve the problem of reducing the dissolution rate, easy increase of moisture in the pharmaceutical composition, inability to ensure product stability and Dissolution and other problems, to achieve the effect of improving compliance, improving drug compliance, and improving the advantages of clinical use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
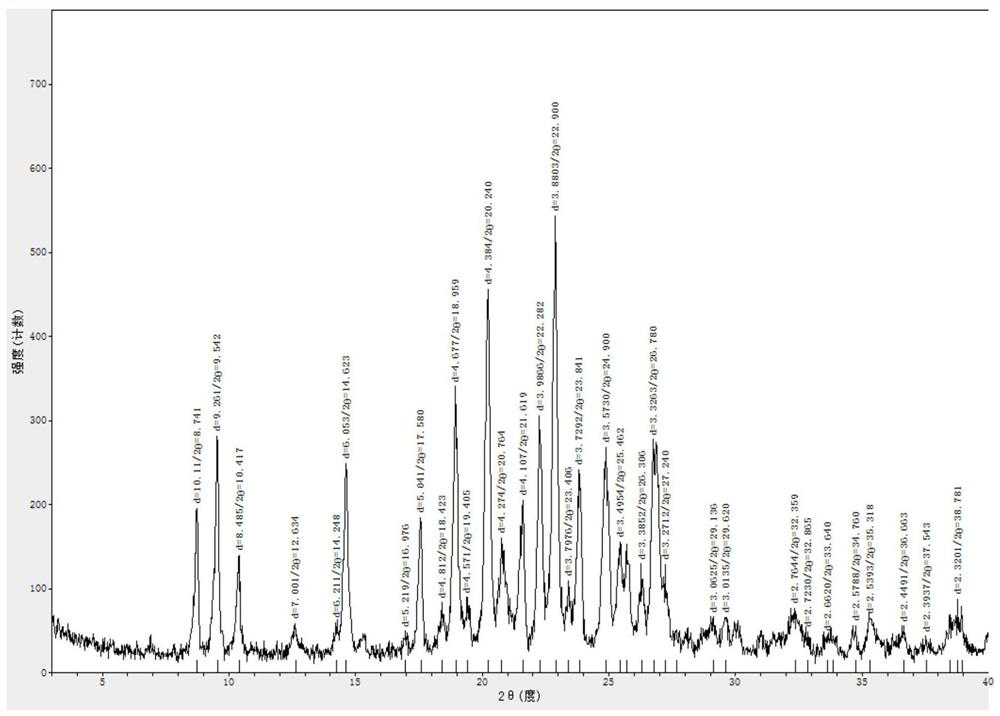
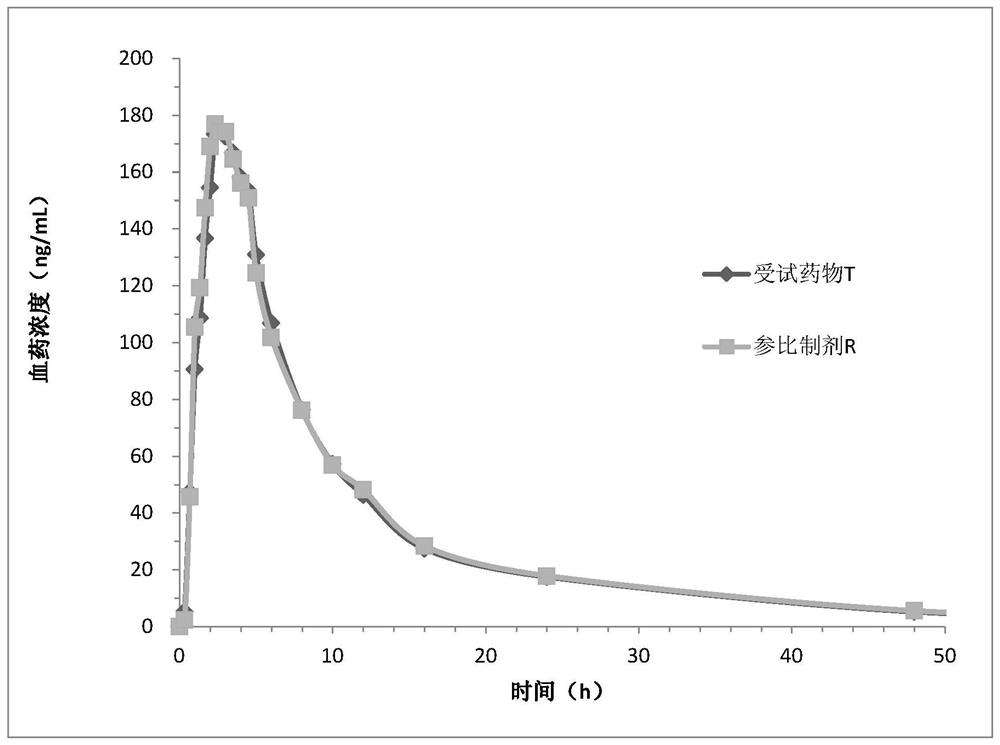
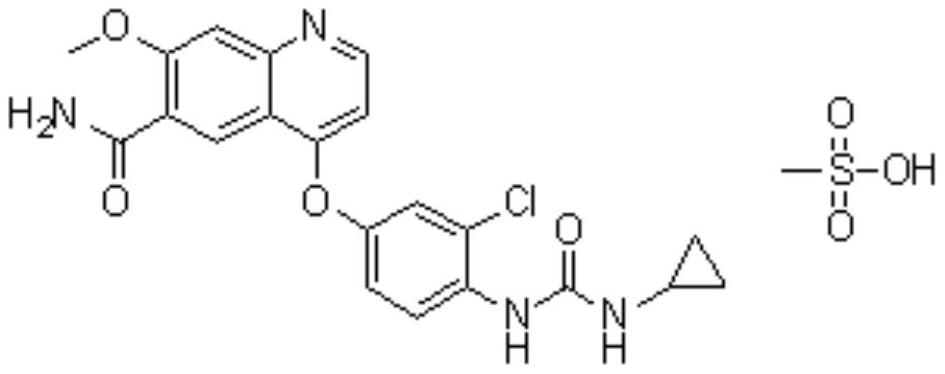
Image
Examples
Embodiment 1-7
[0059] Embodiment 1-7 prescription is as follows:
[0060]
[0061]
Embodiment 1-5
[0062] Embodiment 1-5 preparation technology:
[0063] Prepare lenvatinib mesylate, aluminum magnesium carbonate, microcrystalline cellulose (2 / 3 amount), mannitol, and low-substituted hydroxypropyl cellulose in the above prescription ratios, mix well and use high-speed stirring to prepare Granulation device, adding hydroxypropyl cellulose aqueous solution for wet granulation. The obtained wet granules were dried at 60° C., sieved and sized. Add talcum powder and microcrystalline cellulose (1 / 3 amount) to the sized granules according to the prescription ratio in the above table, and mix well. The granules obtained are respectively filled in No. 2 hard capsules (Example 2, 3) or No. 3 hard capsules (Example 1, 4, 5, 6, 7), and are packaged to prepare capsules containing the composition of the present invention agent.
Embodiment 6-7
[0064] Embodiment 6-7 preparation technology:
[0065] Prepare lenvatinib mesylate, aluminum magnesium carbonate, pregelatinized starch (2 / 3 amount), lactose, and low-substituted hydroxypropyl cellulose in the above prescription ratios, mix well and granulate with high-speed stirring device, adding hydroxypropyl cellulose aqueous solution for wet granulation. The obtained wet granules were dried at 60° C., sieved and sized. Add talcum powder and pregelatinized starch (1 / 3 amount) to the sized granules according to the prescription ratio in the above table, and mix well. The obtained granules were respectively filled into No. 3 hard capsules, and packaged to prepare capsules containing the composition of the present invention.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com