Bovine herpes virus type I antibody blocking ELISA detection method
A bovine herpes virus and detection method technology, which is applied in the directions of viral peptides, chemical instruments and methods, botanical equipment and methods, etc., can solve the problem that the bovine herpes virus type I detection method cannot perform fast, high-throughput, detection, and inability to detect virus particles and other problems, to achieve the effect of high specificity, low emission and time saving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0026] Specific embodiment one: the detection method of bovine herpes virus type I antibody blocking ELISA of the present embodiment is carried out according to the following steps:
[0027] 1. The antigen recombinant gD protein coated by the ELISA plate is coated with the reaction plate, and BSA is blocked;
[0028] 2. After the serum to be tested and the negative and positive control serum are processed, add the reaction plate to block;
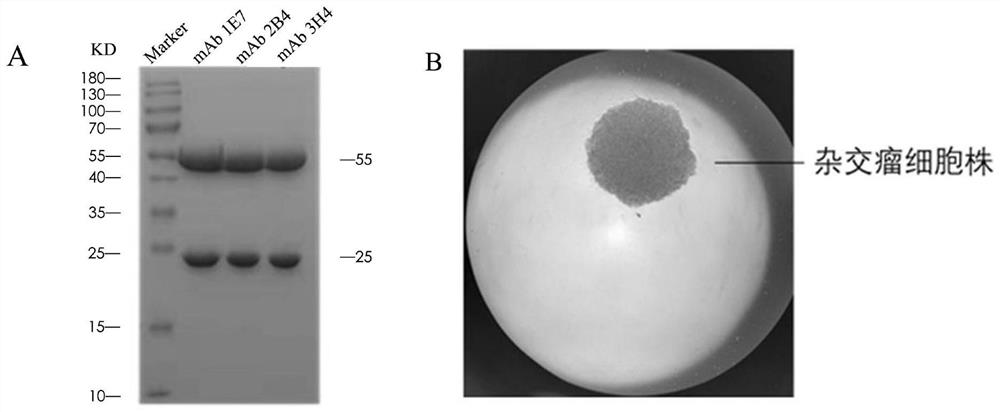
[0029] 3. Horseradish peroxidase-labeled mAb 2B4 binds to the unblocked antigen gD protein, and develops color;
[0030] 4. Terminate the reaction, measure OD450nm, and calculate the inhibition rate; that is, the detection is completed.
[0031] In the first step of this embodiment, the preparation method of the BSA blocking solution is as follows:
[0032] 1. First prepare PBS with pH 7.4: weigh 8g NaCl, 0.2g KCl, 1.42g Na 2 HPO 4 ·12H 2 O and 0.27gKH 2 PO 4 Add 800mL ddH 2 Stir until all dissolved, adjust pH to 7.4, dilute to 1L, ...
specific Embodiment approach 2
[0053] Embodiment 2: The difference between this embodiment and Embodiment 1 is that the antigenic recombinant gD protein in step 1 is prepared according to the following method:
[0054] 1. Extracting bovine herpesvirus type I genomic DNA;
[0055] 2. Recovery and purification of amplified PCR products, primer sequences are as follows:
[0056] gD-T1: 5'-G GAATTC ATGCAAGGGCCGACATTGG-3’
[0057] gD-T2: 5'-CC CTCGAG CAGCGCGCTGTAGTTGACGTT-3’
[0058] 3. The PCR product is ligated with the T vector, and the ligated product is transferred into competent cells, identified by enzyme digestion, screened for positive clones, and sequenced;
[0059] Fourth, Eco RI-HF and Xho I double digestion of positive cloned plasmids and pGEX-6P-1, construction of pGEX-6P-1-gD expression vector, transfer into competent cells, pick positive clones after culture, double digestion identification;
[0060] 5. The culture medium of positive clones was induced to express gD recombinant protein wit...
specific Embodiment approach 3
[0064] Embodiment 3: The difference between this embodiment and Embodiment 2 is that: in step 2, the PCR amplification conditions are: 98°C pre-denaturation for 30s; 98°C denaturation for 10s, 63°C annealing for 30s, 72°C extension for 45s, setting 25 cycle, with a final extension at 72°C for 10 min. Other steps and parameters are the same as in the second embodiment.
[0065] In this embodiment, the temperature and time of pre-denaturation, denaturation, annealing, and extension are set according to the high GC content PCR enzyme instructions of Beijing Sizhengbai Biotechnology Co., Ltd.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com