F-Ketamine hapten, F-Ketamine antigen as well as preparation method and application thereof
A technology of fluoroamine and hapten, which is applied in the biological field, can solve the problems that cannot meet the requirements of rapidity, convenience, and accuracy, and cannot meet the detection standard of fluoroamine, and achieves the reduction of cross-substance interference, mild process conditions, and easy operation. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] This embodiment prepares a kind of fluoroamine hapten, and the preparation method is as follows:
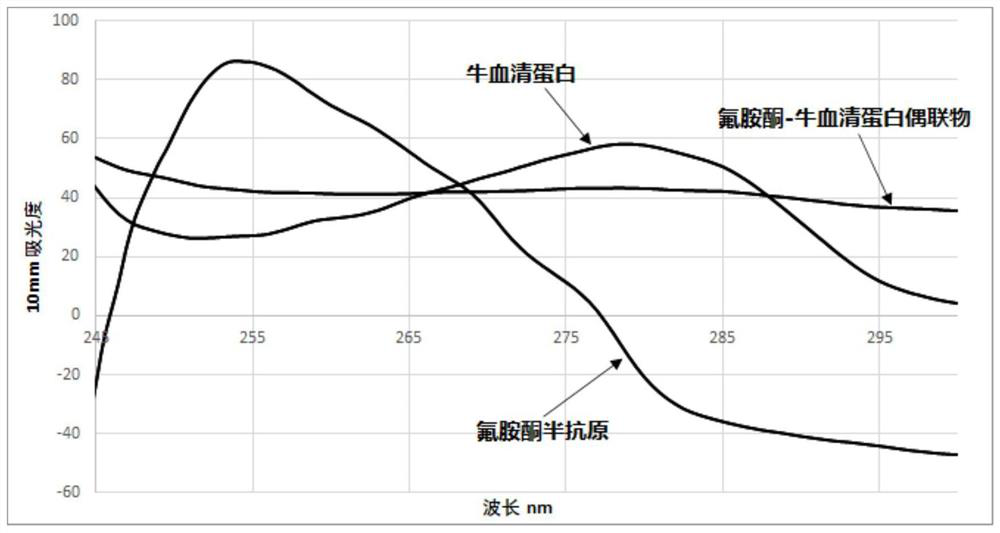
[0066] Weigh 10mmol carboxymethyl hydroxylamine hydrochloride and 10mmol K 2 CO 3 In a 50mL round-bottomed flask, add 21mL methanol and 9mL purified water, stir well for 20min, add 0.1mmol 18-crown-6 and 10mmol fluoroamine, and reflux at 65°C for 24h; after the reaction, evaporate under reduced pressure Dry, add 50mL ultrapure water and 50mL dichloromethane to the residue, separate the water phase and adjust the pH to 4 with 2M hydrochloric acid, extract twice with 10mL n-butanol, combine the n-butanol layers and carry out dry and vacuum distillation to obtain 2.3 The mmol hapten is ready for use; the prepared hapten is analyzed by ESI-MS (295.1 [M+1]), the result is as follows figure 1 As shown, it was proved that the hapten of fluoroamine was successfully prepared.
Embodiment 2
[0068] In this example, a fluoroamine antigen is prepared, and the preparation method is as follows:
[0069] (1) Preparation of Fluoramine hapten active ester:
[0070] In a 25mL dry single-necked bottle, add 1mmol of the hapten prepared in Example 1, 1.2mmol of N-hydroxysuccinimide, 1.2mmol of cyclohexylcarbodiimide and 8mL of N,N-dimethylformamide. Stir at 25°C for 18h under the protection of helium. After the reaction was completed, the reaction mixture was divided into eight 1.5mL centrifuge tubes, centrifuged at 10000rpm for 30min, and the supernatant was divided into eight 2mL glass sealed bottles and stored under the protection of helium gas for later use.
[0071] (2) Preparation of fluoroamine antigen (fluoroamine-bovine serum albumin):
[0072] Take 100mg of BSA in a 25mL one-mouth bottle, add 8mL of 0.1M phosphate buffer with pH 7.0 and stir to dissolve, use an ice-water bath to keep the temperature of the reaction mixture at about 4°C, and slowly add 2.6mL of ac...
Embodiment 3
[0079] This embodiment prepares an immunochromatographic diagnostic reagent strip and reagent card for detecting fluoroamine in urine samples, and the preparation method is as follows:
[0080] (1) Sample pad preparation: Soak the glass fiber in the sample pad treatment solution, and then dry it. The sample pad treatment solution is a formula of 0.02M pH 8.4 Tris and contains the following components by mass fraction: 0.8% Triton X-100, 0.8 %Tween-80, 2% rabbit serum, 1% HPMC, 1% trehalose, 1% LowCross-Buffer, 2% NaCl, 1% Na 2 CO3 , 0.5‰Proclin 300.
[0081] (2) Marking pad preparation: take the mass fraction of 0.01% HAuCl 4 100mL of aqueous solution, add 0.75mL of 1% trisodium citrate aqueous solution by mass fraction, heat and boil for 30min, the color turns red. Turn off the heat and let cool for later use. Colloidal gold solution with 0.1MK 2 CO 3 Adjust the pH to 9.0, stir the colloidal gold solution, add anti-flutamine monoclonal antibody (provided by Guangzhou Wan...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com