Germacrane type sesquiterpene lactone compound, and preparation method and application thereof
A technology of ester compounds and sesquiterpenes, which is applied in the field of gemane-type sesquiterpene lactones and their preparation, can solve the problems of single target, adverse reactions, and narrow treatment range, and achieve the purpose of inhibiting osteoclasts The effect of cell differentiation and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
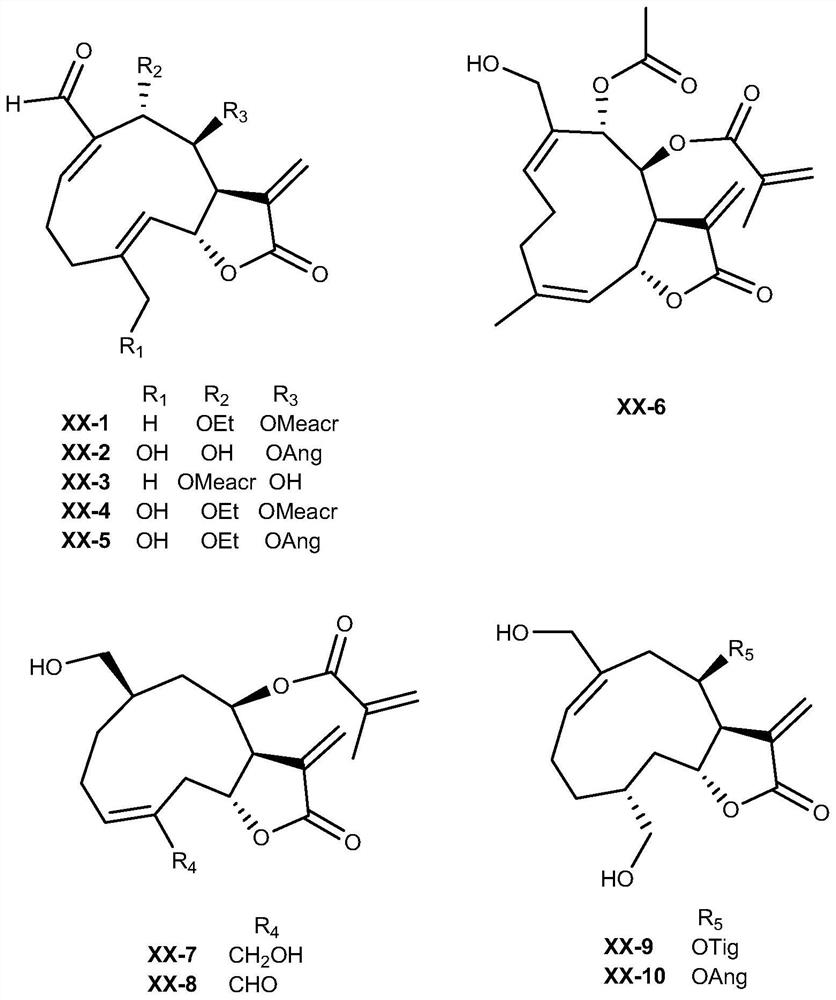
[0040] XX-1 to XX-10 were prepared from C. adenoides.
[0041] The dry aerial part (5.0kg) of Glandularia spp., crushed into small pieces, was extracted with 95% ethanol aqueous solution at room temperature for 3 times, each time for 3 days; the extract was concentrated under reduced pressure to obtain the crude extract; The paste was suspended in water, extracted 5 times with ethyl acetate, concentrated under reduced pressure to obtain the ethyl acetate fraction, which was chromatographed on a 200-300 mesh silica gel column, and eluted with dichloromethane / methanol according to a gradient. The volume ratio of dichloromethane / methanol in gradient elution is 0→40min: 1:0→100:1, 40→80min: 100:1→50:1, 80→120min: 50:1→20:1, 120→160min: 20:1→10:1. After identification by thin-layer chromatography, it was divided and concentrated into 5 fractions (A~E). Fraction C (50 g) was subjected to reverse-phase column chromatography and eluted with methanol / water (30%-100%) to obtain compou...
Embodiment 2
[0060] Determination of anti-osteoporotic activity of compounds.
[0061] 1. Cell culture
[0062] Two types of cells were used in this experiment: primary mouse bone marrow macrophages (Bone Marrow Macrophage, BMM) and RAW264.7 mononuclear macrophages.
[0063] (1) Isolation and culture of BMM cells: 8-week-old female C57BL / 6 mice were sacrificed by cervical dislocation, soaked in 75% ethanol for 10 minutes, and then transferred to an ultra-clean workbench to dissect and separate the tibia and femur of both hind limbs. Remove excess muscle tissue and soak in α-MEM medium containing 1% double antibody for 5 minutes. The joints at both ends of the bone were cut open and placed in α-MEM complete medium containing 10% fetal bovine serum (FBS) and 1% double antibody. Blow out the bone marrow cells with a 1 mL syringe and filter the bone marrow cells with a cell strainer with a diameter of 40 μM. Transfer the cell suspension to a centrifuge tube, centrifuge at 1000rpm for 10min,...
Embodiment 3
[0088] According to the method of Example 1, the formulas (I), (II), (III) and (IV) were first prepared, and water for injection and Tween 80 were added as usual, finely filtered, potted and sterilized to make an injection.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| degree of unsaturation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com