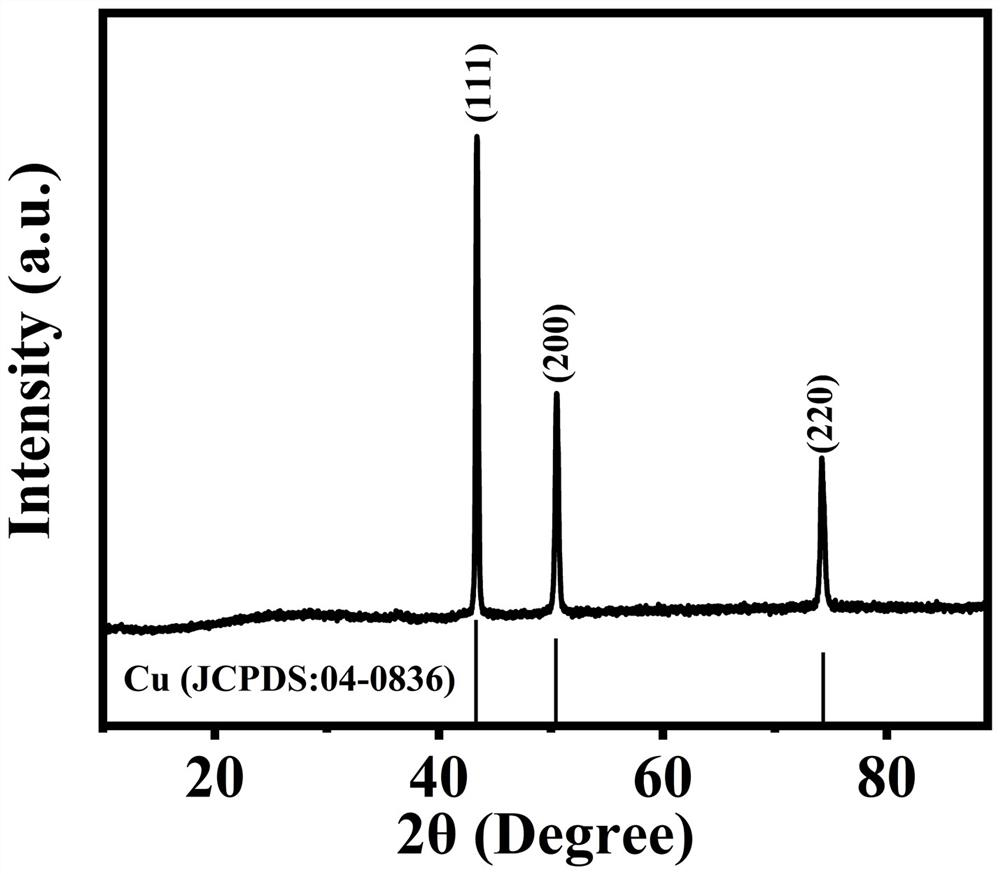
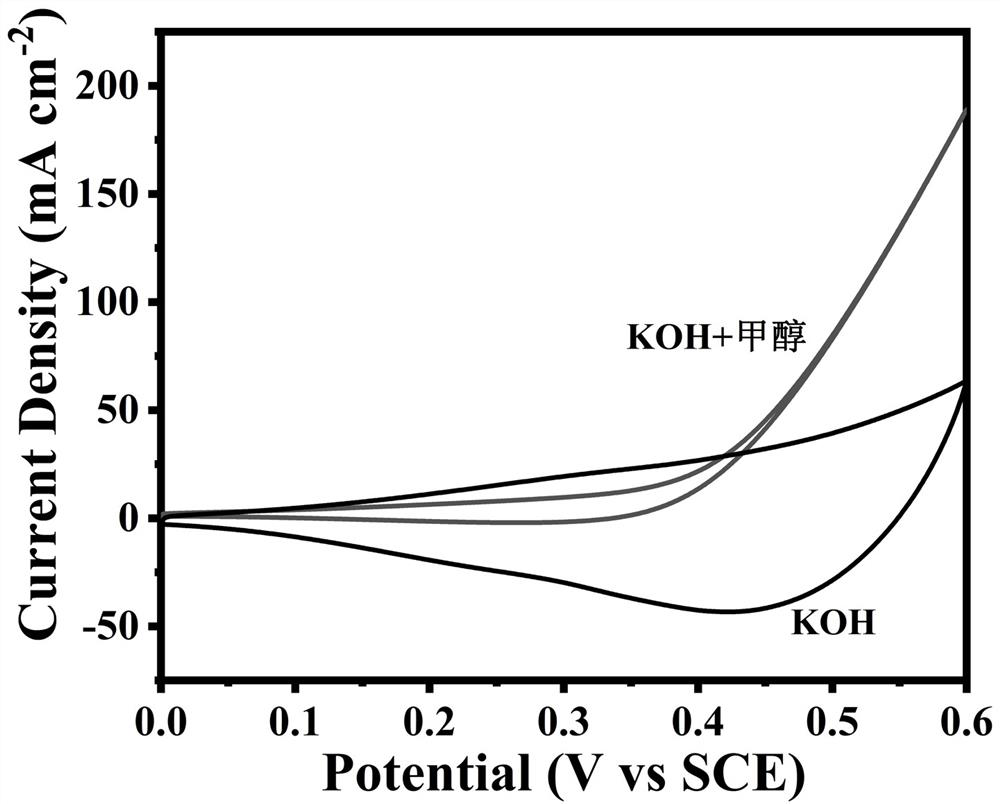
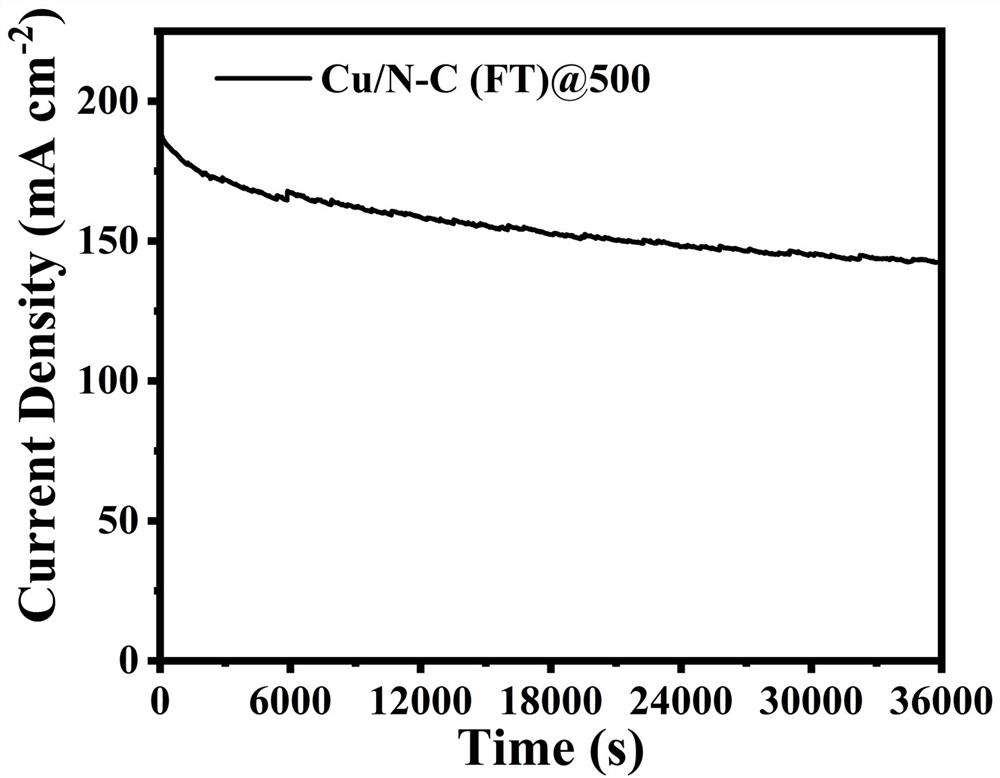
Copper-based anode catalyst for direct methanol fuel cell, and preparation method thereof
A methanol fuel cell and catalyst technology, applied in fuel cells, battery electrodes, electrochemical generators, etc., can solve problems affecting the electrocatalytic performance of methanol oxidation, uneven dispersion of active component copper, and restrictions on wide application, etc., to achieve excellent Effects of electrochemical reactivity, avoidance of inactive regions, and enhancement of catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Weigh 0.0303g (0.175mmol) of triaminophenyl borate hydrochloride (ABA), and dissolve it in 835µL of 6mol / L hydrochloric acid solution to prepare an ABA solution.
[0035] Add the above ABA solution, 0.233g (2.5mmol) aniline (AN) and 62µL deionized water into 2mL 10wt% polyvinyl alcohol (PVA) solution in turn, heat and stir in a water bath at 55°C for 30min, until the solution is clear, place it on ice Cool in a water bath to 0°C, add 1.375mL 2mol / L ammonium persulfate (APS) solution dropwise, stir quickly and evenly, pour it into a mold, and let it stand at room temperature for 24h to prepare polyvinyl alcohol-polyaniline conductive hydrogel polymer film.
[0036] Place the formed polyvinyl alcohol-polyaniline conductive hydrogel polymer film in a refrigerator at -20°C for 8 hours, take it out, let it thaw at room temperature, wash it with deionized water, and place it in a solution containing 0.1mol / L H 2 SO 4 In the 1mol / L copper sulfate electroplating solution, the...
Embodiment 2
[0040] Weigh 0.0358g (0.21mmol) of triaminophenyl borate hydrochloride (ABA), and dissolve it in 835µL of 6mol / L hydrochloric acid solution to prepare an ABA solution.
[0041] Add the above ABA solution, 0.264g (2.8mmol) aniline (AN) and 234µL deionized water into 2mL 10wt% polyvinyl alcohol (PVA) solution in turn, heat and stir in a water bath at 55°C for 30min, until the solution is clear, place it on ice Cool in a water bath to 0°C, add 1.652mL 2mol / L ammonium persulfate (APS) solution dropwise, stir quickly and evenly, pour it into a mold, and let it stand at room temperature for 24 hours to prepare polyvinyl alcohol-polyaniline conductive hydrogel polymer film.
[0042] The formed polyvinyl alcohol-polyaniline conductive hydrogel polymer film was placed in a -20°C refrigerator for 8 hours, taken out, and thawed at room temperature. After 3 freeze-thaw cycles, wash with deionized water and place in a place containing 0.1mol / L H 2 SO 4 In the 0.5mol / L copper sulfate ele...
Embodiment 3
[0045] Weigh 0.0182g (0.11mmol) of triaminophenyl borate hydrochloride (ABA), and dissolve it in 835µL of 6mol / L hydrochloric acid solution to prepare an ABA solution.
[0046] Add the above-mentioned ABA solution, 0.189g (2.0mmol) aniline (AN) and 710µL deionized water into 2mL 10wt% polyvinyl alcohol (PVA) solution in turn, heat and stir in a water bath at 55°C for 30min, until the solution is clear, place on ice Cool in a water bath to 0°C, add 0.937mL 2mol / L ammonium persulfate (APS) solution dropwise, stir quickly and evenly, pour it into a mold, and let it stand at room temperature for 24h to prepare polyvinyl alcohol-polyaniline conductive hydrogel polymer film.
[0047] The formed polyvinyl alcohol-polyaniline conductive hydrogel polymer film was placed in a -20°C refrigerator for 8 hours, taken out, and thawed at room temperature. After 5 freeze-thaw cycles, wash with deionized water and place in a 2 SO 4 In the 3mol / L copper sulfate electroplating solution, the po...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com