Copper-cobalt-zinc composite self-supporting nano array electrode material and preparation method and application thereof
An electrode material and nano-array technology, which is applied in the field of copper-cobalt-zinc composite self-supporting nano-array electrode material and its preparation, can solve the problems of inherent energy barrier and slow electron transfer, limited reserve hindering, increasing interface resistance, etc. Electrochemical reactivity, green reaction, and the effect of reducing interface resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] The invention provides a method for preparing a copper-cobalt-zinc composite self-supporting nano-array electrode material (method for short), characterized in that the method comprises the following steps:
[0028] Step 1. Oxidize copper to copper hydroxide by oxidation-reduction reaction, then wash and dry to obtain a regular and orderly copper hydroxide nanowire array structure (referred to as a wire array structure);
[0029] Preferably, in step 1, the copper is copper foam (CF).
[0030] Preferably, in step 1, the oxidation-reduction reaction is to soak the copper into the oxidation-reduction agent until the solid matter changes from copper yellow to blue.
[0031] Preferably, the redox agent is NaOH and (NH 4 ) 2 S 2 o 8 Aqueous solution of NaOH and (NH 4 ) 2 S 2 o 8 The mol ratio is 20:1~5 (preferably 20:1), soaking time is 10~30min (preferably 20min);
[0032] Preferably, in step 1, the washing is to wash with water several times before washing with eth...
Embodiment 1
[0049] Step 1, placing foam copper in 20:1 NaOH and (NH 4 ) 2 S 2 o 8 Soak in the aqueous solution for 20 minutes, oxidize the foamed copper to copper hydroxide by oxidation-reduction reaction, then take it out and wash it with ethanol solution to neutral pH=7, dry it in a vacuum oven at 60°C for 3 hours to obtain a linear array structure;
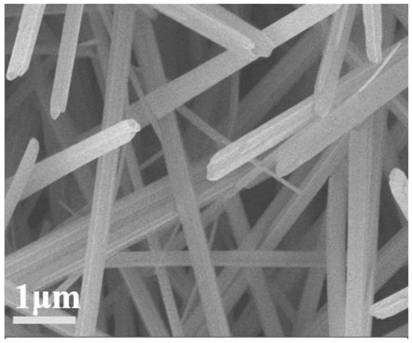
[0050] Depend on figure 1 It can be seen that the linear array structure obtained in step 1 is uniform and arranged in an orderly manner, with a diameter ranging from 250 to 350 nm and a length of 5 to 10 μm.
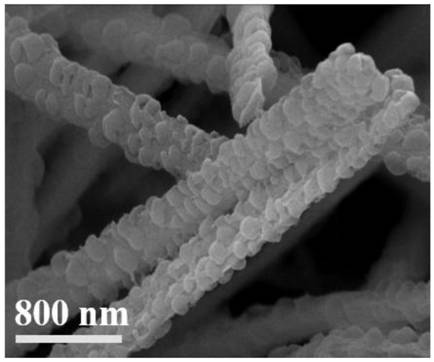
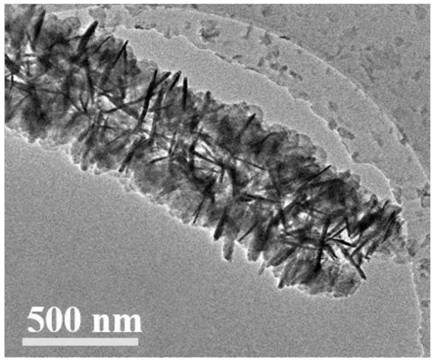
[0051] Step 2, the product that step 1 obtains is soaked in containing Co(NO 3 ) 2 ·6H 2 O and Zn(NO 3 ) 2 ·6H 2 O molar ratio is 1h in the methanol solution of 2:8, then is placed in the 2-methylimidazole methanol solution of 0.4M, slowly stirs 30min; The mol ratio of metal ion and 2-methylimidazole is 1:8; Then Take it out and wash it with methanol solution to neutral pH = 7, and dry it in a vacuum oven at 100°C for 8 ho...
Embodiment 2
[0054] Step 1, placing foam copper in 20:1 NaOH and (NH 4 ) 2 S 2 o 8 Soak in the aqueous solution for 20 minutes, oxidize the foamed copper to copper hydroxide by oxidation-reduction reaction, then take it out and wash it with ethanol solution to neutral pH=7, dry it in a vacuum oven at 60°C for 3 hours to obtain a linear array structure;
[0055] Step 2, the product that step 1 obtains is soaked in containing Co(NO 3 ) 2 ·6H 2 O and Zn(NO 3 ) 2 ·6H 2 O molar ratio is the methanol solution of 4:6 1h, then is placed in the 2-methylimidazole methanol solution of 0.4M, slowly stirs 30min; The mol ratio of metal ion and 2-methylimidazole is 1:8; Then Take it out and wash it with methanol solution to neutral pH = 7, and dry it in a vacuum oven at 100°C for 8 hours to obtain a self-supporting structure @ZIF;
[0056] Step 3, the product that step 2 obtains is placed in containing Co(NO 3 ) 2 ·6H 2 O and Zn(NO 3 ) 2 ·6H 2 O in an ethanol solution with a molar ratio of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com