Novel antigen peptide composition and application thereof in tumor immunotherapy drugs
A technology of immunotherapeutic drugs and antigenic peptides, which is applied in the fields of molecular immunology and biomedicine, and can solve problems such as low accuracy of neoantigens, unsatisfactory immunogens, and damage to normal tissue cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
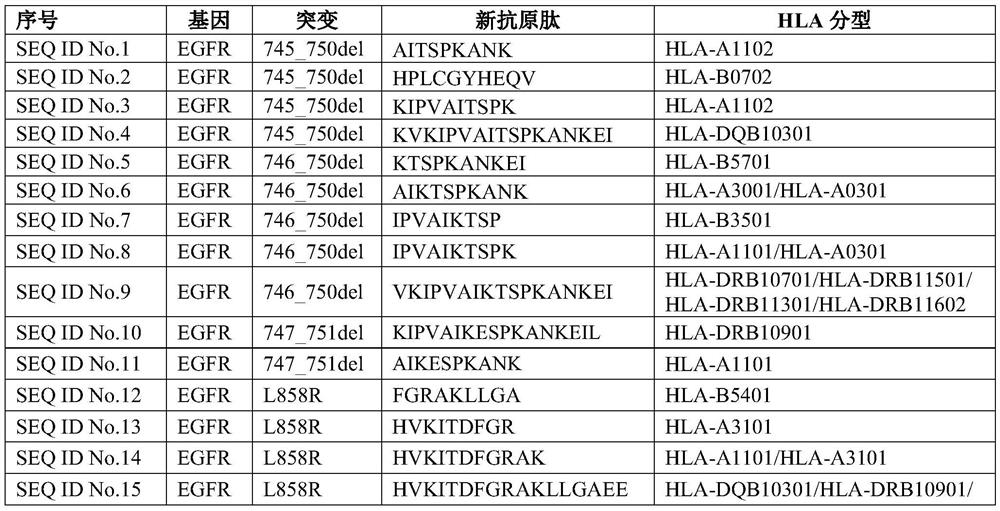
[0049] Example 1 New Antigen Peptide Immune Function Verification
[0050] 1. Isolation of Peripheral Blood Mononuclear Cells (PBMCs)
[0051] Take 20ml of venous blood from a healthy donor with the corresponding HLA type, dilute the blood sample with an equal volume of sterile PBS, transfer the lymphocyte separation solution (Stemcell, 07861) into a new 50ml centrifuge tube, and ensure that the blood volume ratio is 3 : 4. Carefully add the diluted blood to the surface of the separation liquid, operate as gently as possible, avoid mixing, and clearly see the boundary between the two liquids. Centrifuge at 400g for 30min at room temperature. After centrifugation, suck out the mononuclear cell layer, transfer it to a new sterile centrifuge tube, and add PBS to wash twice. Cells were resuspended in RPMI-1640 medium and counted.
[0052] 2. Enzyme-linked immunospot (ELISPOT) verification of peptide immune response
[0053] Will 2.5×10 6 Each cell was suspended in 90% RPMI-16...
Embodiment 2
[0057] Example 2 Neoantigen peptide activates specific T lymphocytes
[0058] 1. Isolation and induction of DC cells
[0059] T cells and monocytes were isolated from PBMCs of healthy donors typed HLA-A1101, and monocytes were first enriched using a human CD14 positive selection kit (STEMCELL, 18058). T cells were then isolated from CD14- cells using a human T cell isolation kit (STEMCELL, 17951). Monocytes were suspended in X-VIVO-15 medium and adjusted to 1 × 10 6 / mL, and then treated with GM-CSF (100ng / mL) and IL-4 (50ng / mL). Incubate cells at 37 °C, 5% CO 2 cultured for 6 days to induce immature DC, and then TNF-α (10ng / mL), IL-1β (10ng / mL), IL-6 (100ng / mL), LPS (1μg / mL) and PGE2 (1μg / mL) for 24 hours to induce DC maturation.
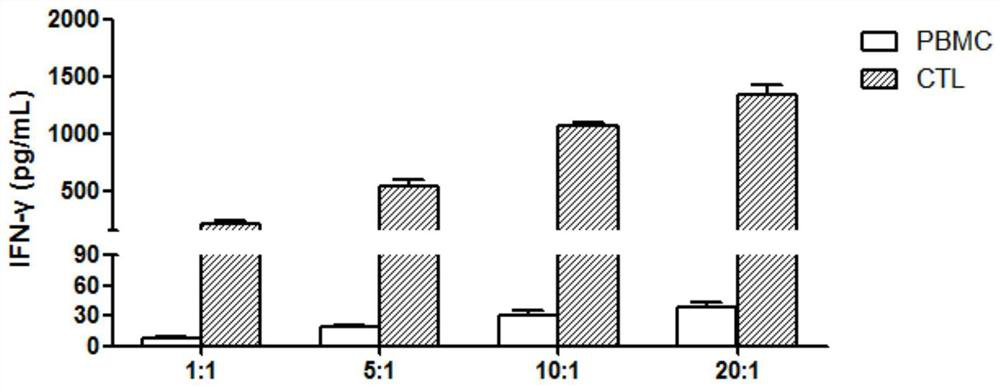
[0060] 2. Induction of specific cytotoxic T lymphocytes (CTLs) by DCs loaded with neoantigen peptide compositions
[0061] Will 2.5×10 5 DC cells with 1×10 6 T cells were mixed, and IL-2 (50ng / mL), IL-7 (5ng / mL), IL-15 (10ng / mL) and the ne...
Embodiment 3
[0064] Example 3 In vivo anti-tumor experiment of specific T lymphocytes activated by neoantigen peptide composition
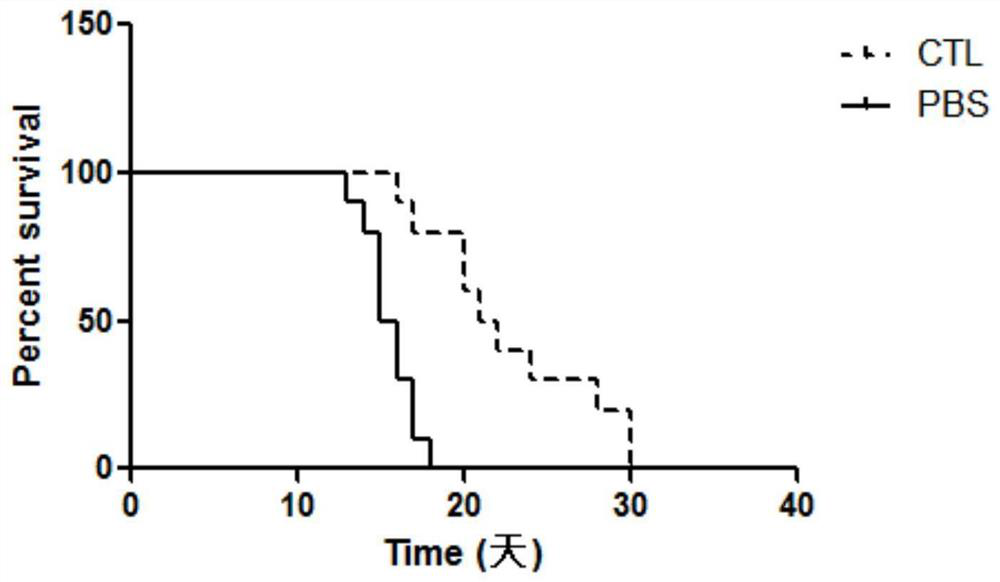
[0065] Take the A1101-H2172 cells in the logarithmic growth phase, the cell density is about 80-90%, digest the cells with trypsin, wash twice with pre-cooled PBS, and adjust the cells to an appropriate concentration. Take 20 6-week-old nude mice, weighing 18-20g, and subcutaneously inoculate 1×10 7 Cells were observed to form tumors, and nude mice with a tumor diameter of 5 mm were selected and randomly divided into two groups. The treatment group was given the CTL cells prepared in Example 2, and injected 1×10 7 cells / time, 2 times a week for 3 consecutive weeks. The control group was given PBS solution. The tumor size was measured every week, the tumor volume was calculated, and the survival rate of the two groups was observed. The result is as figure 2 As shown, compared with the control group, the tumor survival rate of the CTL cell treatment group ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com