Squaramide-bisbenzimidazole conjugates with pH-dependent anion transmembrane transport activity, and methods for their synthesis
A technology of bisbenzimidazole and transmembrane transport, applied in the fields of drug combination, organic chemistry, antitumor drugs, etc., can solve the problems of large molecular weight, complex drug synthesis, low antitumor activity, etc., and achieve the effect of promoting transmembrane transport
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] This embodiment provides the synthesis of compound 14-26
[0044]
[0045] Compound 14: Weigh N-Boc-gly (805mg, 4.59mmol), EDC (1.77g, 9.23mmol), HOBt (936mg, 6.92mmol) and DMAP (30mg, 0.24mmol) into a 100mL reaction flask, and use CH 2 Cl2 (40mL) dissolved;
[0046] After stirring at room temperature for 10 min, the mixture was transferred to a dropping funnel, and slowly (1 drop / 6s) was added dropwise to a solution of o-phenylenediamine (500 mg, 4.62 mmol) and DMF (1.5 mL);
[0047] The resulting mixed solution was stirred and reacted at room temperature for 3 days. After the mixed solution was removed under reduced pressure, ethyl acetate (20 mL) was added, and saturated NaCl aqueous solution (20 mL×2), saturated NH 4 Cl aqueous solution (20mL×2) and saturated NaHCO 3 After washing with aqueous solution (20 mL×2), it was washed with saturated aqueous NaCl solution (20 mL). use Na 2 SO 4 After drying, ethyl acetate was removed under reduced pressure;
[0048]...
Embodiment 2
[0062] This example provides the synthesis of compounds 27-29
[0063]
[0064] Compound 27: Weigh compound 14 (60mg, 0.24mmol) in a 25mL reaction vial, 3After OH (3 mL) was dissolved, HCl (2M, 3 mL) was added and stirred at room temperature for 1 h. After the solvent was removed under reduced pressure, aqueous ammonia (3 mL×2) was added to the reaction flask, stirred at room temperature for 15 min, and then the aqueous ammonia was removed under reduced pressure. Add methanol (2mL) to dissolve, separate by column chromatography (dichloromethane / methanol / ammonia, 24 / 3 / 1, v / v / v), and freeze-dry to obtain compound 27 (39mg, 45%). 1 H NMR (500 MHz, MeOD) δ 7.54 (dd, J=6.0, 3.2 Hz, 2H), 7.22 (dd, J=6.0, 3.2 Hz, 2H), 4.05 (s, 2H).
[0065] Compound 28: Synthesized similarly to Compound 27, 40 mg, 72%. 1 H NMR (CD 3 OD, 400MHz) δ7.30(d, J=8.2Hz, 1H), 7.21(s, 1H), 6.95(d, J=8.2Hz, 1H), 3.91(s, 2H), 2.34(s, 3H) .
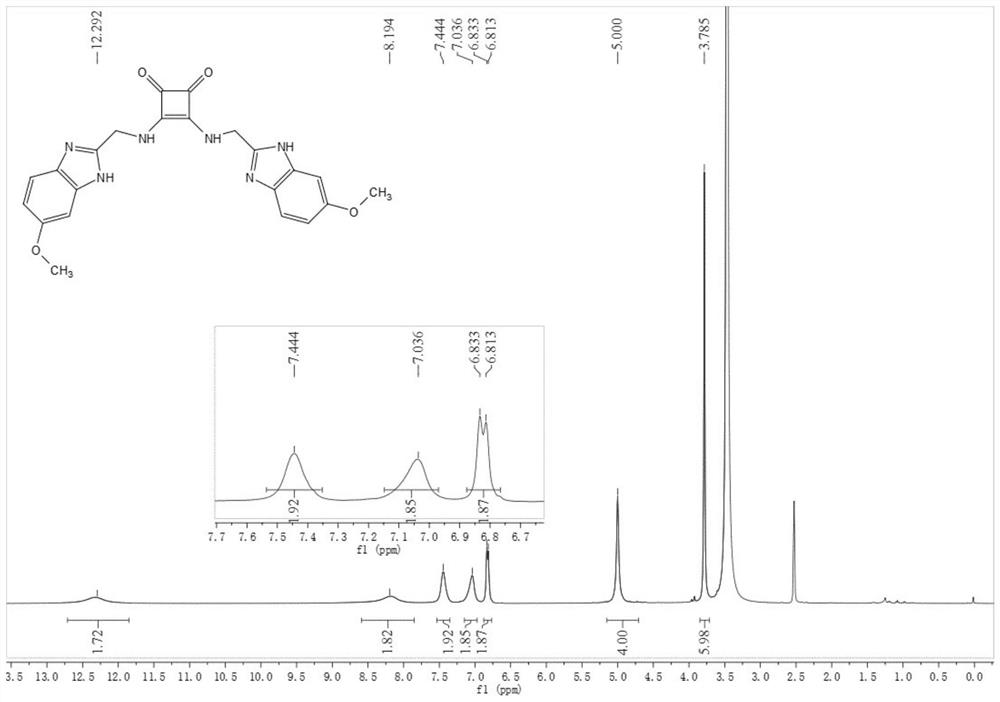
[0066] Compound 29: Synthesized similarly to Compound 27, 55 m...
Embodiment 3
[0078] Synthesis of compounds 1-13
[0079]
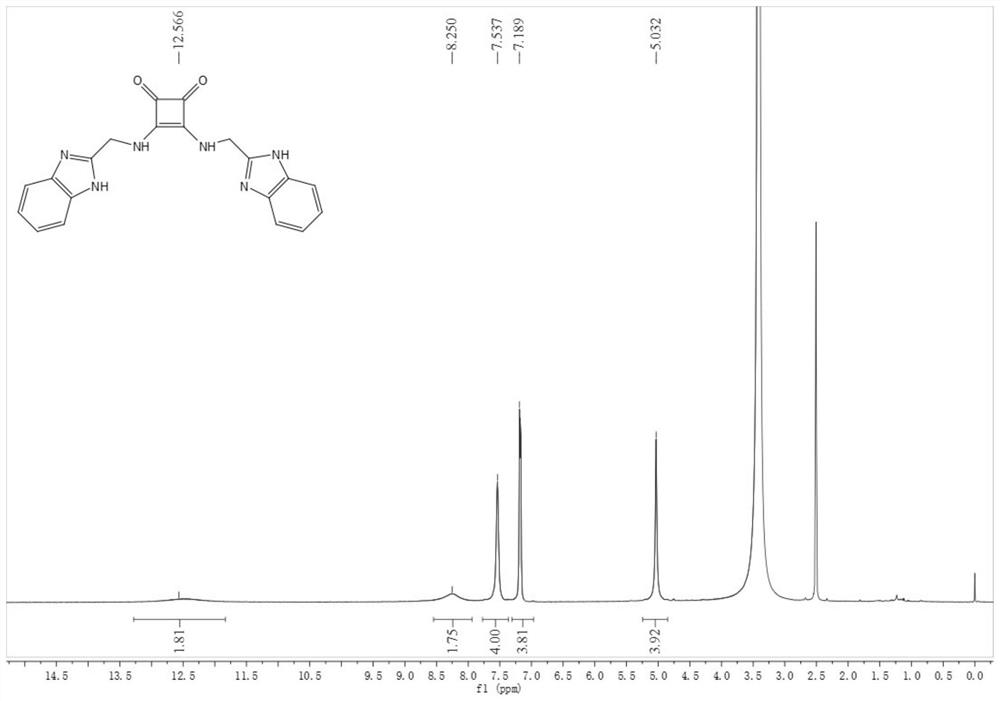
[0080] Compound 1: Dissolve compound 27 (39 mg) in ethanol (3 mL), then add triethylamine (300 μL), react at room temperature for 30 minutes, add 3,4-diethoxy Cyclobut-3-ene-1,2-dione (21 mg, 0.12 mmol) in ethanol (270 μL). After reacting at room temperature for 23 h, suction filtration under reduced pressure gave a white solid, compound 1 (12 mg, 31%). 1 H NMR (DMSO-d 6 , 400MHz, see figure 1 )δ12.57(s,2H),8.25(s,2H),7.54(s,4H),7.19(s,4H),5.03(s,4H); 13 C NMR (DMSO-d 6 ,100MHz)δ183.4,168.2,151.9,122.2,115.9,115.4; negative ESI-MS: m / z 371.07 ([M-H] – ) and negative HR-ESI-MS for C 20 h 16 N 6 o 2 ([M-H] – ) Calcd: 371.12510, Found: 371.12570.
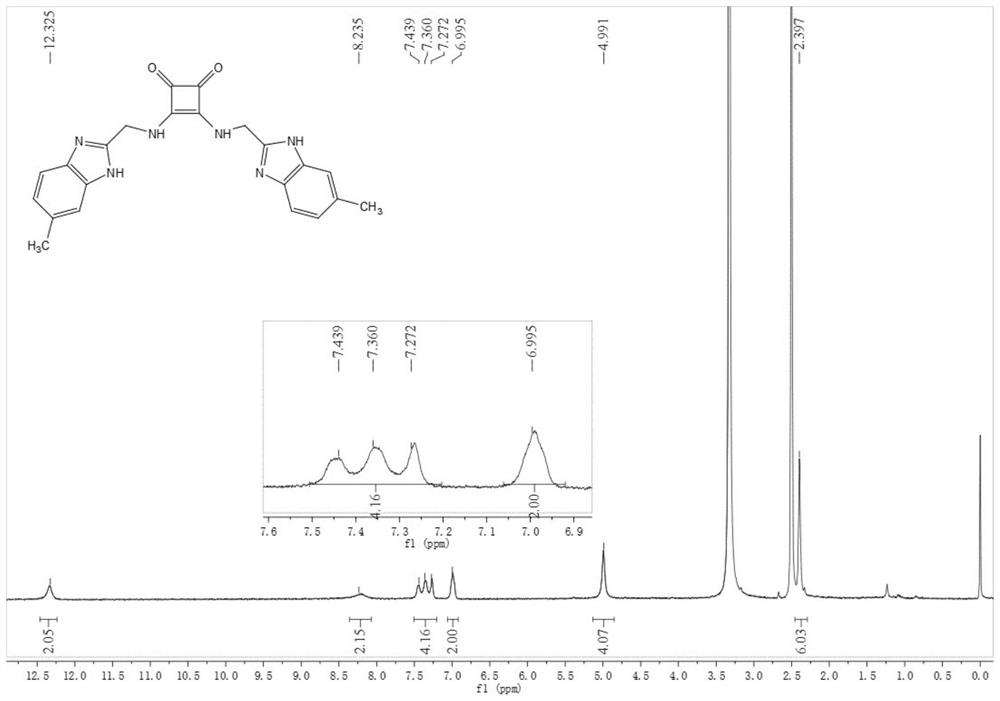
[0081] Compound 2: Synthesized similarly to Compound 1, 35 mg, 79%. 1 H NMR (DMSO-d 6 , 400MHz, see figure 2 )δ12.33(s,2H),8.21(s,2H),7.51–7.20(m,4H),7.00(s,2H),4.99(s,4H),2.40(s,6H); 13 CNMR (DMSO-d 6 ,100MHz) δ183.3, 168.2, 132.3, 111.5, 21.6; negativeESI-MS: m / z 399.0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com