Application of bifidobacterium lactis tci604 and its metabolites
A TCI604, Bifidobacterium lactis technology, applied in the direction of bifidobacteria, applications, metabolic diseases, etc., can solve problems such as complications and patient disease recurrence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1: Protease activity detection test of Bifidobacterium lactis TCI604
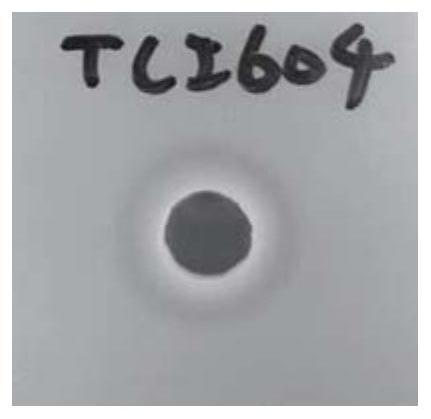
[0054] The cryopreservation tube containing the Bifidobacterium lactis TCI604 strain of [Preparation Example A-3] was thawed, and the TCI604 strain was inoculated into an MRS medium (BDTMDifcoTM Lactobacilli MRS Broth) and cultured at 37°C to activate the strain . Next, take a piece of Skim milk agar and dig a hole in it. Then, 50 microliters of the once activated TCI604 strain was injected into the holes on the aforementioned agar and incubated at 37°C for 24 hours. Finally, observe whether a transparent ring is produced on the cultured agar (if a transparent ring is produced, it means that the cultured strain has protease activity), and take pictures to record. Results are shown in figure 1 .
[0055] Depend on figure 1 It can be seen that Bifidobacterium lactis TCI604 of the present invention can form a transparent ring on skim milk agar. The aforementioned results show that the Bifi...
Embodiment 2
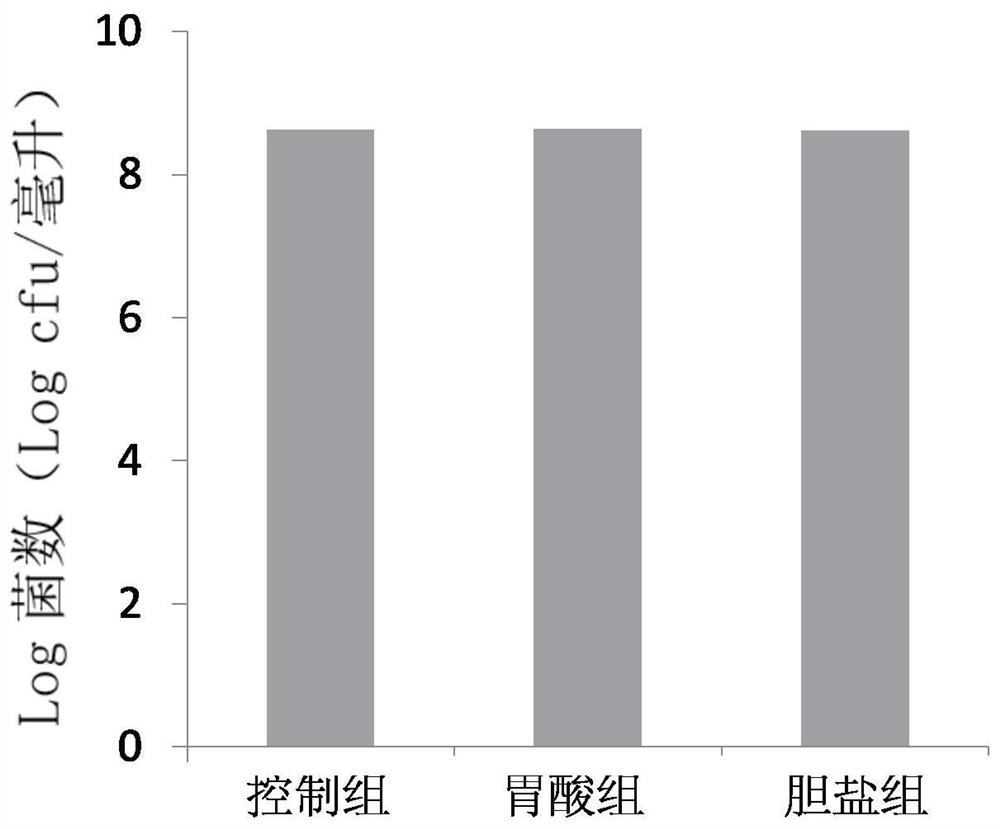
[0056] Example 2: Gastric acid resistance and bile salt resistance test of Bifidobacterium lactis TCI604
[0057] The cryopreservation tube containing the Bifidobacterium lactis TCI604 strain of [Preparation Example A-3] was thawed, and the TCI604 strain was inoculated into an MRS medium (BDTMDifcoTM Lactobacilli MRS Broth) and cultured at 37°C to activate the strain . The activated TCI604 strain was then rinsed twice with 1x phosphate-buffered saline (Phosphate-bufferedsaline, PBS), and then re-dissolved with 0.3 ml of PBS (total bacterial count was about 1x10 10 ). Divide the lysate after the aforementioned treatment into three groups (each group contains 0.1 ml of the lysate), and add each group of lysates to the following solutions:
[0058] 1. Control group: 99 ml of 0.2M KCl solution (pH 7).
[0059] 2. Gastric acid group: 99 ml of 0.2M KCl solution (pH 1.2).
[0060] 3. Bile salt group: 99 ml of 0.2M KCl solution (containing 0.3% bile salt).
[0061] Next, the solu...
Embodiment 3
[0063] Example 3: Intestinal colonization effect test of Bifidobacterium lactis TCI604
[0064] (3-1) The human colon adenocarcinoma cell line Caco-2 was cultured in a triangular dish (T75Flask) for 120 hours. Next, the culture medium was removed, and after rinsing the cells twice with 1x PBS, 1 ml of 1% trypsin (Trypsin / EDTA) was added to suspend the cells. The aforementioned cell suspension was injected into a 24-well plate (4 × 10 5 cells / well) at 37°C, 5% CO 2 Incubate overnight. After that, after rinsing the cells twice with 1×PBS, 900 μl of fresh antibiotic-free culture medium was added to each well.
[0065](3-2) Thaw the cryopreservation tube containing the Bifidobacterium lactis TCI604 strain of [Preparation Example A-3], inoculate the TCI604 strain into MRS medium (BDTMDifcoTMLactobacilli MRS Broth) and culture at 37°C, to activate the strain. The activated TCI604 strain was then rinsed twice with 1x PBS, and then re-solubilized with 1x PBS (total bacterial coun...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com