Application of siRNA targeting FGF5 in the treatment of hair loss
A hair loss and composition technology, applied in the field of screening these substances, can solve the problem of low transfection efficiency of siRNA
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Design, synthesis and cholesterol modification of embodiment 1siRNA
[0040] According to the mRNA sequence of mouse FGF5 in Gene Bank, siRNA design was performed using online open software. Because different design software uses different algorithms, the design principles also have their own emphasis. As an example, to improve the accuracy of siRNA design, we compared the characteristics of each software, and finally selected 3 kinds of software with strong technical support and continuous updating. The software SiDirect, Oligowalk and SiRNA Design are used for siRNA design, and siRNAs with high repetition rates are selected from the output sequences as candidate siRNAs for further cell biology experiment screening to confirm their gene interference activity.
[0041] The synthesis of candidate siRNAs and the covalent modification of cholesterol at the 5' end were entrusted to Shanghai Gemma Pharmaceutical Technology Co., Ltd.
Embodiment 2
[0042] Example 2 Screening of siRNA and evaluation of target gene interference effect of different siRNA delivery methods
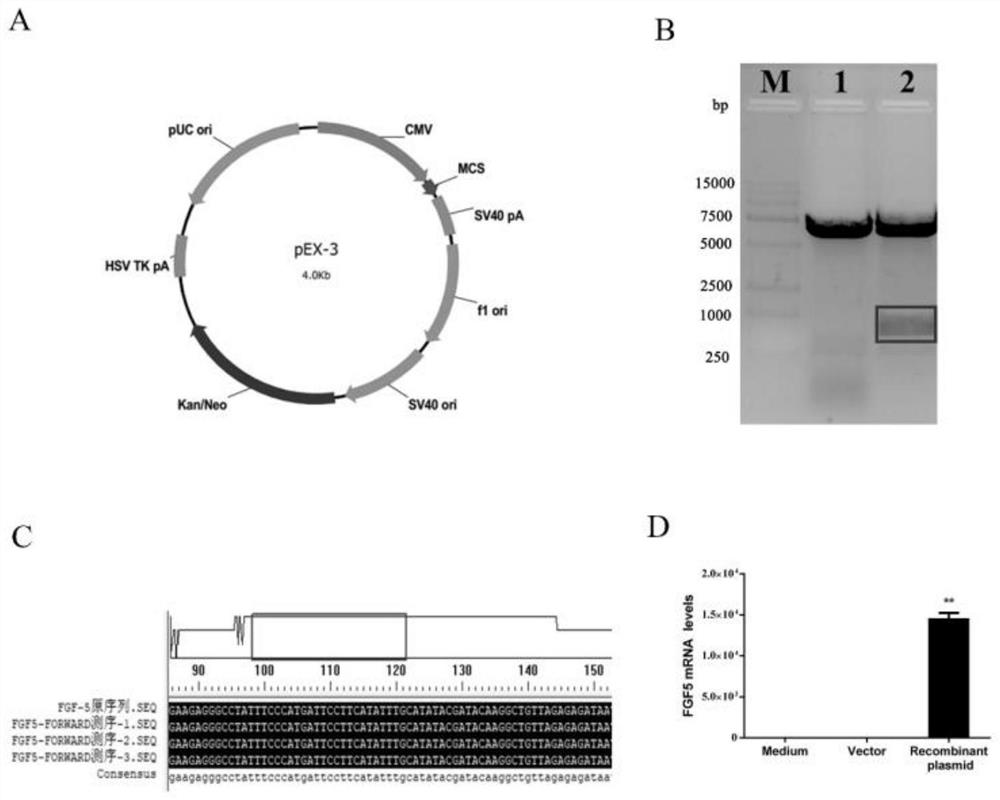
[0043] Since the cell line expressing FGF5 is currently unavailable, in this study, the eukaryotic expression plasmid of mouse FGF5 was constructed, transfected into NIH / 3T3 cells, and the NIH / 3T3 cells overexpressing mouse FGF5 were used to screen candidate siRNAs. The eukaryotic expression plasmid of mouse FGF5 can be prepared according to conventional methods in the art.
[0044] (1) NIH / 3T3 cells that have been transfected with the expression plasmid of FGF5 were treated with 1×10 5 The density of cells / well was inoculated into 6-well plate, cultured in 2ml of complete medium containing serum and double antibody, when the cell density reached 80%, replaced with 2ml of serum-free cell culture medium;
[0045] (2) Take 200 pmol of siRNA and dilute it in 100 μl of serum-free and double-antibody-free medium, add 4 μl of lipofectamine 2000 to another 10...
Embodiment 3
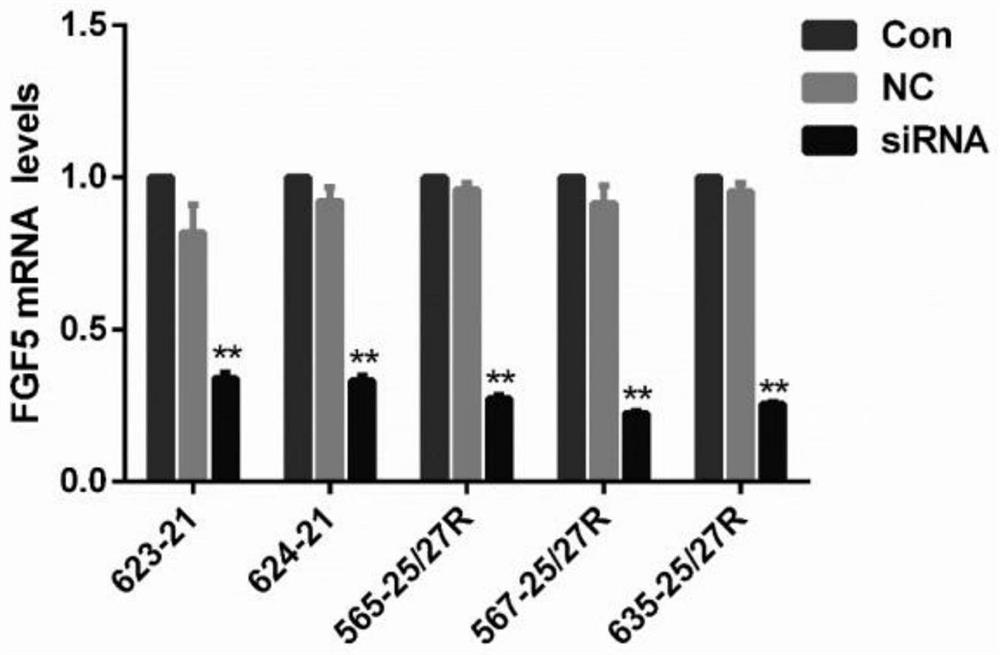
[0048] Example 3 Screening of siRNA transfection delivery system
[0049] 1. Cholesterol-modified siRNA transfection
[0050] (1) NIH / 3T3 cells that had been transfected with FGF5 expression plasmid were treated with 1×10 5 The density of cells / well was inoculated into 6-well plates, cultured in 2ml of cell culture medium containing serum and double antibody, when the cell density reached 80%, replace with fresh complete medium;
[0051] (2) Dilute 200 pmol of cholesterol-modified siRNA (entrusted to Gemma Gene Co., Ltd. to modify according to conventional technical means in the field) in 200 μl of serum-free and double-antibody-free medium, mix gently, let stand for 5 minutes, and add to 6 wells plate, placed in 5% CO 2 , cultured in a cell incubator at 37°C for 24 hours;
[0052] (3) The cells were collected, and the mRNA level of the FGF5 gene in the cells was detected by qPCR.
[0053] 2. Penetrating peptide-mediated siRNA transfection
[0054] (1) NIH / 3T3 cells tha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com