A kind of preparation method of thiabendazole intermediate
A technology for thiabendazole and intermediates, applied in the field of preparation of thiabendazole intermediates, can solve the problems of high price of pyruvic acid, difficult operation, unsafe process and the like, and achieves low cost of raw and auxiliary materials, low cost of raw materials, and low cost of raw materials. The effect of route novelty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
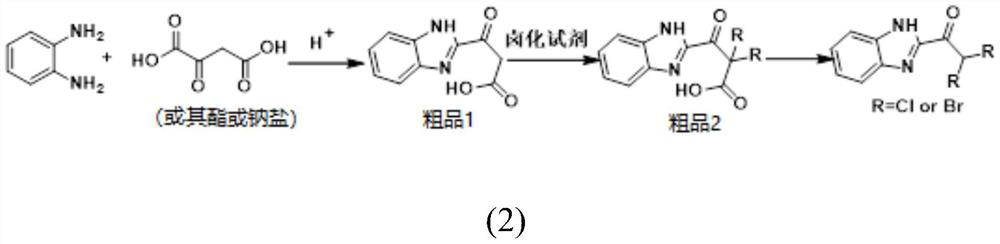
[0031] Under the condition of room temperature (10~35℃), add 110.3g (1mol, 98%, Bailingwei Technology Co., Ltd.) oxaloacetic acid 133.7g (1.1mol, 98%, Bailingwei Technology Co., Ltd.) Company) mixed in dilute hydrochloric acid, heated at 50-70°C for condensation reaction, reaction time 5-6h, after cooling, adjust pH between 7-8 with 30% aqueous sodium hydroxide solution, temperature controlled not higher than 35°C, continue Stir for 30min, separate out solid, cool below 15°C, filter, wash the filter residue with water, and dry 201.8g of crude product 1 at about 50°C, the effective content is 91%, and the yield is 90% in terms of o-phenylenediamine; the crude product 1-201.8 g into a 1000ml four-necked flask, add 500g of dichloroethane, heat at about 40°C, pass in chlorine gas to carry out chlorination reaction, the reaction time is 4-5h, the liquid phase detection of the crude product is 1<1%, the reaction is terminated, and the solvent is recovered at normal pressure Then, cr...
Embodiment 2
[0033] Under the condition of room temperature (10~35℃), add 110.3g (1mol, 98%, Bailingwei Technology Co., Ltd.) diethyl oxaloacetate 211.2g (1.1mol, 98%, o-phenylenediamine) into a 1000ml four-necked flask, Bailingwei Technology Co., Ltd.) mixed in dilute hydrochloric acid, heated at 50-70°C for condensation reaction, reaction time 5-6h, after cooling, adjust pH between 7-8 with 30% aqueous sodium hydroxide solution, and the temperature should not be higher than 35 ℃, continue to stir for 30min, separate out solid, cool below 15 ℃, filter, wash the filter residue with water, 201.8g of crude product 1 after drying at about 50 ℃, the effective content is 91%, and the yield is 90% in terms of o-phenylenediamine; Add 1-201.8g into a 1000ml four-necked flask, add 500g of dichloroethane, heat at about 40°C, pass in chlorine gas to carry out chlorination reaction, the reaction time is 4-5h, the liquid phase detection of crude product 1<1%, terminate the reaction, usually After the s...
Embodiment 3
[0037]Under the condition of room temperature (10~35℃), add 110.3g (1mol, 98%, Bailingwei Technology Co., Ltd.) oxaloacetic acid 133.7g (1.1mol, 98%, Bailingwei Technology Co., Ltd.) Company) mixed in dilute hydrochloric acid, heated at 50-70°C for condensation reaction, reaction time 5-6h, after cooling, adjust pH between 7-8 with 30% aqueous sodium hydroxide solution, temperature controlled not higher than 35°C, continue Stir for 30min, separate out solid, cool below 15°C, filter, wash the filter residue with water, and dry 201.8g of crude product 1 at about 50°C, the effective content is 91%, and the yield is 90% in terms of o-phenylenediamine; the crude product 1-201.8 g into a 1000ml four-necked flask, add 500g of dichloroethane, heat at about 40°C, pass in chlorine gas to carry out chlorination reaction, the reaction time is 4-5h, the liquid phase detection of the crude product is 1<1%, the reaction is terminated, and the solvent is recovered at normal pressure Then, cru...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com