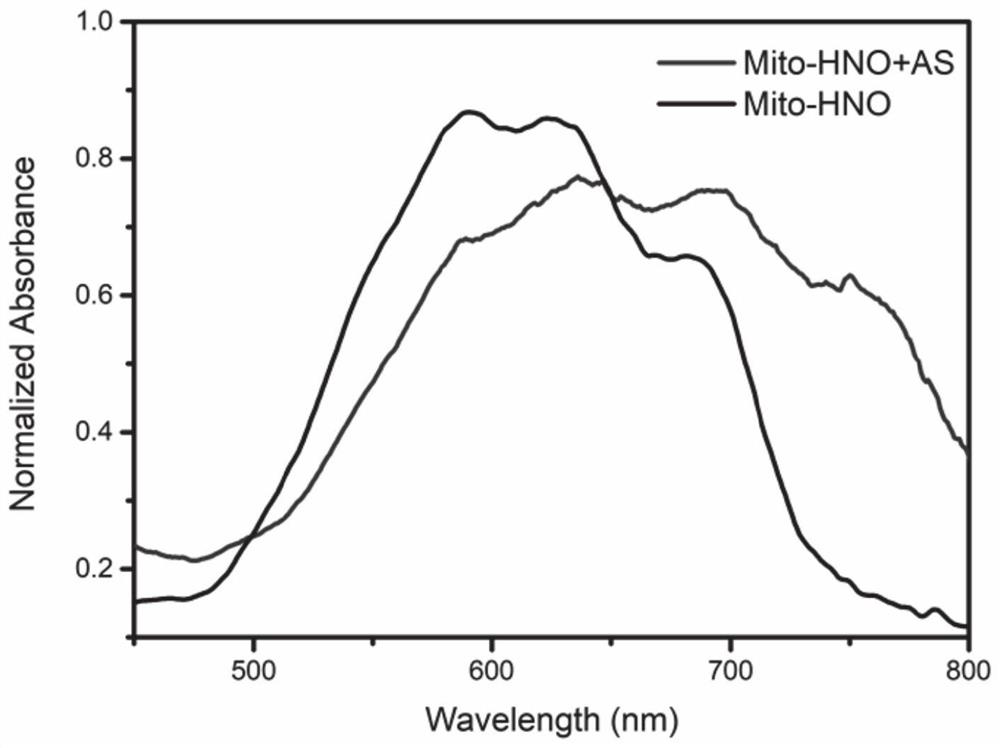
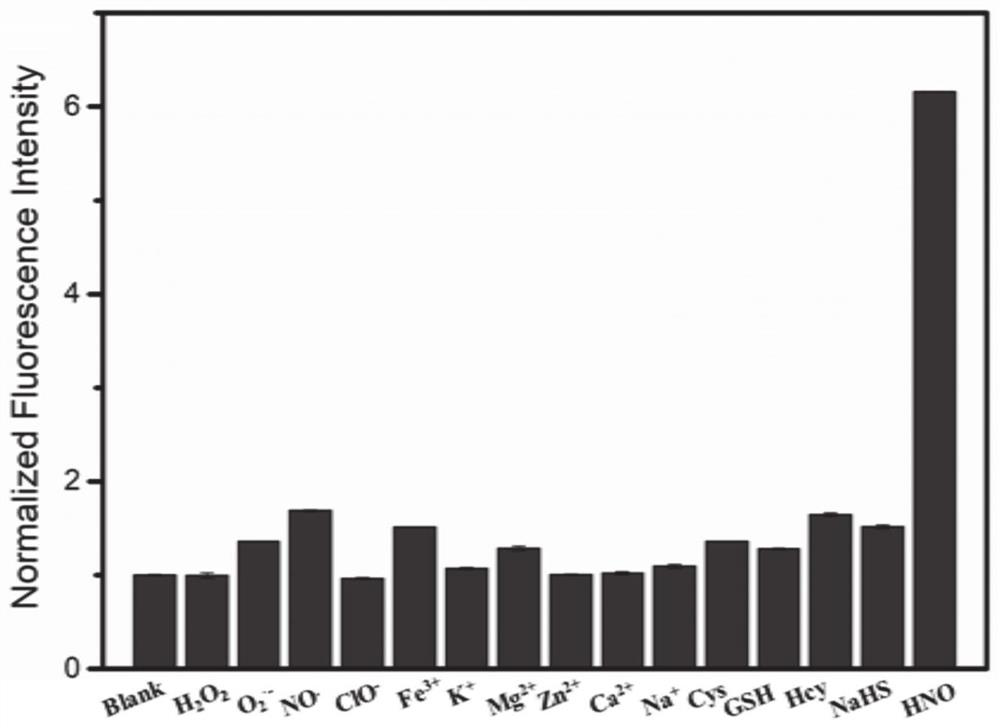
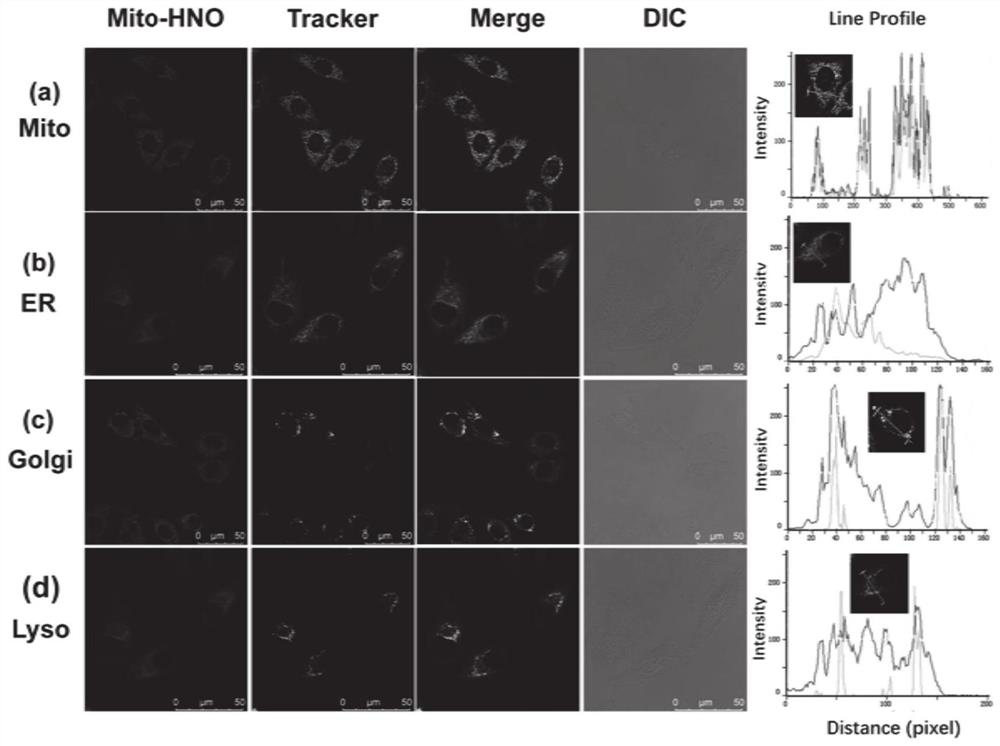
Fluorescent probe Mito-HNO, preparation method thereof and application of fluorescent probe Mito-HNO in detection of HNO in mitochondria
A fluorescent probe and reaction technology, applied in chemical instruments and methods, luminescent materials, pharmaceutical formulations, etc., can solve problems such as short time, difficult capture, and limitations, achieve deep tissue penetration depth, facilitate in vivo imaging, and design strategies and the effect of the simplicity of the synthetic route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046]Synthesis of fluorescent probes
[0047] The synthesis of hydroxy merocyanine (substance 4) was synthesized according to the existing reports. Dissolve the raw material 2-diphenylphosphobenzoic acid (5mmol), dicyclohexylcarbodiimide (5mmol), and 4-dimethylaminopyridine (0.25mmol) in 15mL of dichloromethane, activate the carboxyl group at 0°C for 30min, Hydroxy merocyanine (1 mmol) was then added and stirred at room temperature for 12 h. After the reaction was complete, the solvent was removed by rotary evaporation. Then using dichloromethane:methanol=20:1 as eluent, purified by column chromatography to obtain Mito-HNO (57%) as a blue-purple solid.
[0048] NMR and mass spectrometry characterization:
[0049] 1 H NMR (400MHz, CDCl 3 )δ=8.71(d,J=15.2Hz,1H),8.36–8.31(m,1H),8.20(d,J=8.4Hz,1H),8.05(dd,J=18.2,8.5Hz,2H), 7.73–7.66(m,2H),7.62–7.52(m,3H),7.39–7.24(m,12H),7.11(d,J=1.8Hz,1H),7.07–7.05(m,1H),6.83– 6.76(m,2H),4.86(q,J=7.3Hz,2H),2.82(dt,J=63.9,5.7Hz,4H),2.07(s,...
Embodiment 2
[0061] Synthesis of fluorescent probes
[0062] The synthesis of hydroxy merocyanine (substance 4) was synthesized according to the existing reports. Dissolve the raw materials 2-diphenylphosphobenzoic acid (1mmol), dicyclohexylcarbodiimide (1mmol), and 4-dimethylaminopyridine (0.05mmol) in 15mL of dichloromethane, activate the carboxyl group at 0°C for 30min, Hydroxy merocyanine (1 mmol) was then added and stirred at room temperature for 12 h. After the reaction was complete, the solvent was removed by rotary evaporation. Then using dichloromethane:methanol=20:1 as eluent, purified by column chromatography to obtain Mito-HNO (15%) in blue-purple solid.
Embodiment 3
[0064] Synthesis of fluorescent probes
[0065] The synthesis of hydroxy merocyanine (substance 4) was synthesized according to the existing reports. Dissolve the raw material 2-diphenylphosphobenzoic acid (3mmol), dicyclohexylcarbodiimide (3mmol), and 4-dimethylaminopyridine (0.15mmol) in 15mL of dichloromethane, activate the carboxyl group at 0°C for 30min, Hydroxy merocyanine (1 mmol) was then added and stirred at room temperature for 12 h. After the reaction was complete, the solvent was removed by rotary evaporation. Then using dichloromethane:methanol=20:1 as eluent, purified by column chromatography to obtain Mito-HNO (24%) as blue-purple solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com