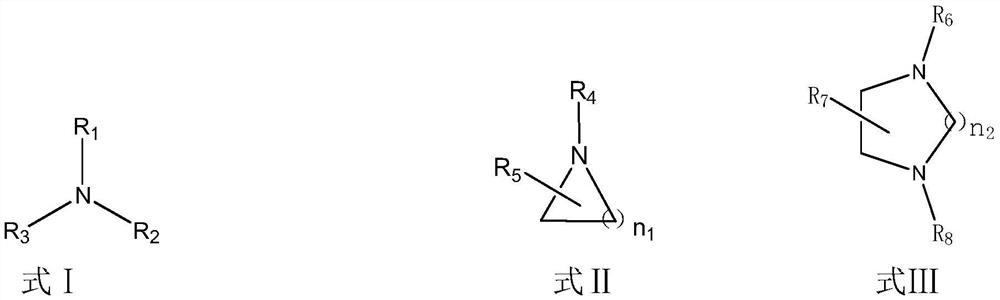
Purification method of dialkyl methylphosphite
A technology of dialkyl methylphosphonite and dialkyl chlorophosphinate is applied in the field of purification of dialkyl methylphosphonite, and can solve the problems of low yield and purity of the purification process, and the like, To achieve the effect of less residue in distillation still, mild reaction conditions, and avoidance of hydrolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
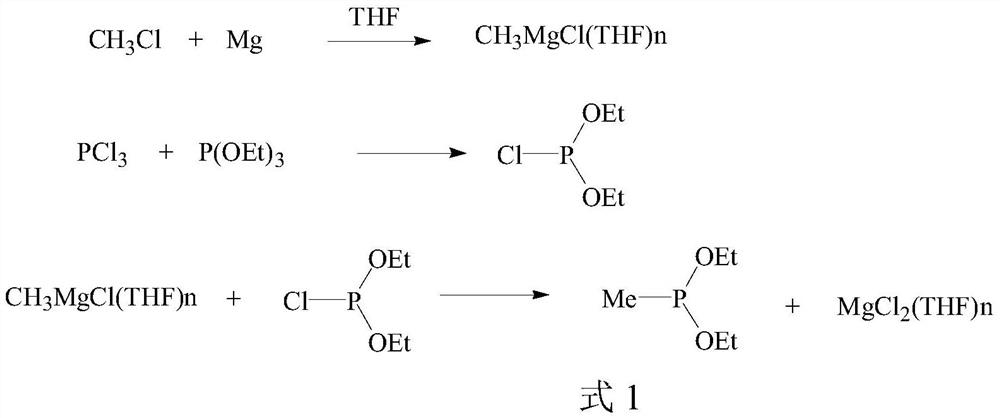
[0042]Preparation of diethyl methyl phosphonite reaction solution:
[0043]Under the protection of nitrogen, add 40mL of tetrahydrofuran and 15.6g (0.1mol) of diethyl phosphonite chloride into a four-neck flask, cool to -10°C, dropwise add 41.1g (0.11) of a tetrahydrofuran solution containing 20wt% methylmagnesium chloride mol), keep the temperature at -10 to 0 DEG C for 2 hours, the content of diethyl chlorophosphinate is less than 0.3% detected by gas chromatography, the reaction is over, and the reaction solution of diethyl methyl phosphonite is obtained.
Embodiment 2
[0045]Preparation of dibutyl methyl phosphonite reaction solution:
[0046] Under the protection of nitrogen, add 40 mL of tetrahydrofuran and 21.3 g (0.1 mol) of dibutyl chlorophosphinate into a four-necked flask, cool to -10°C, and add dropwise 41.1 g (0.11 mol), keep the temperature at -10 to 0° C. and react for 2 hours, the content of dibutyl chlorophosphinate detected by gas chromatography is less than 0.3%, the reaction is over, and the reaction solution of dibutyl methyl phosphonite is obtained.
Embodiment 3
[0048]Purification of diethyl methyl phosphonite:
[0049]Add 9.2g of toluene to the reaction solution of diethyl methylphosphonite obtained in Example 1, add 0.5g of triethylamine, keep the temperature at -10°C, stir slowly for 15h, filter, and dry to obtain a content of over 97% Of anhydrous magnesium chloride. The filtrate was rectified under reduced pressure, and 60-70°C / 50mmHg fractions were collected to obtain 12.2 g of diethyl methylphosphonite, with a gas content of 98.1% and a yield of 87.9%. According to Kjeldahl nitrogen determination method, the nitrogen content in anhydrous magnesium chloride is 0.06%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com