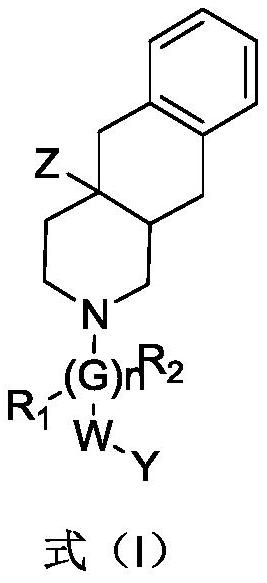
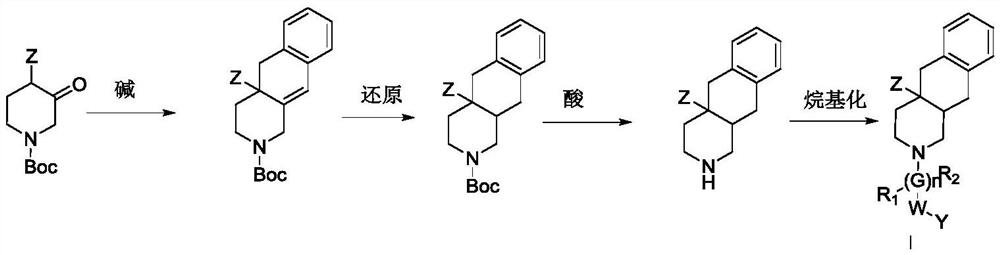
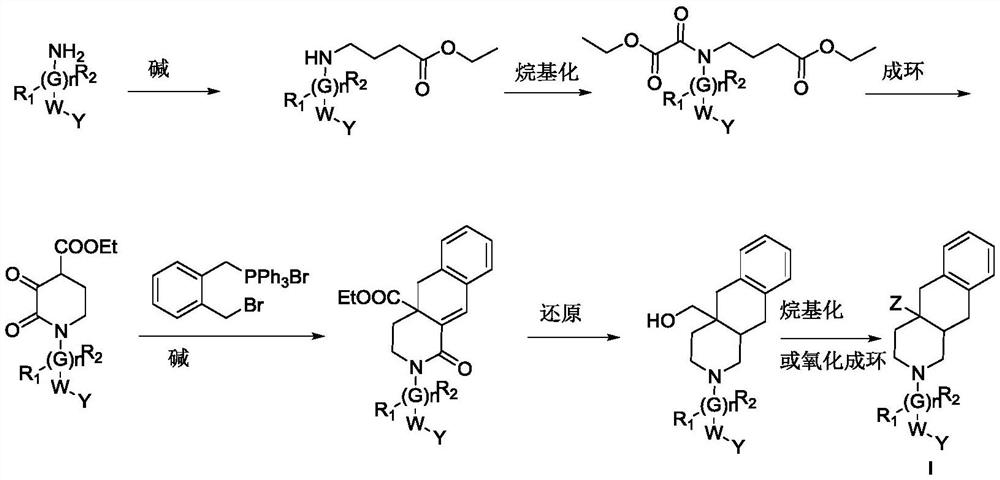
Multi-hydrogen isoquinoline derivative as well as preparation method and medical application thereof
A technology of hydroisoquinoline and derivatives, which is applied in the field of polyhydroisoquinoline derivatives and its preparation, can solve the problems of long cycle, high cost, low success rate, etc., and achieve small side effects, good drug properties, and safety high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Example 1 (6-methylpyridin-3-yl) (4a-(pyridin-2-yl)-3,4,4a,5,10,10a-hexahydrobenzo[g]isoquinoline-2( Synthesis of 1H)-yl)methanone:
[0063]
[0064] 1.1 3-oxo-4-(pyridin-2-yl)piperidine-1,4-dicarboxylic acid 1-(tert-butyl)4-ethyl ester:
[0065] Under nitrogen atmosphere, 1-(tert-butyl) 4-ethyl 3-oxopiperidine-1,4-dicarboxylate (5 g, 18.4 mmol) was dissolved in 100 mL THF, the system was cooled to -78 °C, and 37 mL LHMDS ( 1M) THF solution, stirred for 30min, added 2-fluoropyridine (3.58g, 36.8mmol), continued to react overnight, TLC followed the reaction, after the reaction, added water to the system, and extracted with ethyl acetate (30mL×3 ), the combined organic phases were concentrated and purified by column chromatography to obtain 2.5g yellow viscous product. Yield: 39%. LC-MS (ESI+): m / z 349 (M+1).
[0066] 1.2 tert-butyl 3-oxo-4-(pyridin-2-yl)piperidine-1-carboxylate:
[0067] Under nitrogen atmosphere, 3-oxo-4-(pyridin-2-yl)piperidine-1,4-dicarboxylic...
Embodiment 2
[0076] Example 2 4a-(pyridin-2-yl)-2-(pyridin-3-ylmethyl)-1,2,3,4,4a,5,10,10a-octahydrobenzo[g]isoquine Synthesis of morphine:
[0077]
[0078] Dissolve 4a-(pyridin-2-yl)-1,2,3,4,4a,5,10,10a-octahydrobenzo[g]isoquinoline (40 mg, 0.15 mmol) in 2 mL of DCM, add Nicotinic aldehyde (32.5mg, 0.3mmol), NaBH(OAc) 3 (128.5mg, 0.6mmol) and 0.1mLEt 3 N, stirred at room temperature for 7h, followed the reaction by TLC. After the reaction, 1mL of water was added to the system, and extracted with DCM / MeOH (15 / 1) (2mL×3), the organic phases were combined, washed with saturated brine, Na 2 SO 4 It was dried, concentrated by filtration, and purified by column chromatography to obtain 50 mg of yellow oil, which was converted into citrate to obtain 63 mg. Yield: 90%. LC-MS(ESI+):m / z 356 (M+1); 1H NMR(300MHz,MeOD):δ8.43-8.34(m,3H),7.76-7.74(m,3H),7.36-7.29(m,2H), 7.24-7.20(m,1H),7.17-7.12( m,1H),7.05-7.00(m,1H),6.90-6.87(m,1H),3.52(m,2H), 3.41-3.23(m,2H),2.68-2.57(m,2H),2.41- 2.35(m,...
Embodiment 3
[0079] Example 3 2-((6-methylpyridin-3-yl)methyl)-4a-(pyridin-2-yl)-1,2,3,4,4a,5,10,10a-octahydrobenzene And [g] the synthesis of isoquinoline:
[0080]
[0081] 4a-(Pyridin-2-yl)-1,2,3,4,4a,5,10,10a-Octahydrobenzo[g]isoquinoline (40 mg, 0.15 mmol) was dissolved in 2 mL DCM and added to 6 -Methylnicotinaldehyde (36.7mg, 0.3mmol), NaBH(OAc) 3 (128.5mg, 0.6mmol) and 0.1mLEt 3 N, stirred at room temperature for 7h, followed the reaction by TLC. After the reaction, 1mL of water was added to the system, and extracted with DCM / MeOH (15 / 1) (2mL×3), the organic phases were combined, washed with saturated brine, Na 2 SO 4 It was dried, concentrated by filtration, and purified by column chromatography to obtain 47.1 mg of yellow oil, which was converted into citrate to obtain 62.2 mg. Yield: 81%. LC-MS(ESI+):m / z 370(M+1); 1 H NMR (300MHz, MeOD): δ8.77(br s, 1H), 8.28(s, 1H), 7.78(t, J=7.8Hz, 1H), 7.67(d, J=8.1Hz, 1H), 7.68 -7.32(m,4H),7.26(d,J=6.9Hz,1H),7.16(t,J=7.5Hz,1H),7.02...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com