Application of sophocarpidine in preparation of medicine for treating bacterial vaginosis
A technology of matrine total alkaloids and bacteria, which is applied in the field of matrine total alkaloids in the preparation of drugs for the treatment of bacterial vaginosis, which can solve the adverse effects of difficult inactivation, the inability of the human host immune system to effectively remove pathogens, and healthy vaginal microecology To achieve the effect of improving the cure rate and reducing the recurrence rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Isolation, purification and identification of anaerobic bacteria from vaginal secretions of BV patients
[0025] (1) Criteria for the study population
[0026] Inclusion criteria: ① Bacterial vaginosis patients with Nugent score ≥ 7 and clinical symptoms; ② Age: 18-50 years old; ③ Can complete standard treatment; ④ Informed consent.
[0027] Exclusion criteria: ①Women during pregnancy, lactation and menopause; ②Those who take hormone drugs for a long time due to other diseases; ③Those who take immunosuppressants; ④Those who have heart, liver, kidney, endocrine and other medical diseases (mainly through consultation) ; ⑤ people with trichomonas, vulvovaginal candidiasis and other vaginal infections; ⑥ people allergic to metronidazole; ⑦ people with poor compliance.
[0028] (2) Number of enrolled cases
[0029] 100 patients with BV.
[0030] (3) Isolation, purification and storage of clinical Gardnerella strains
[0031] 1) Put the casman agar plate coate...
Embodiment 2
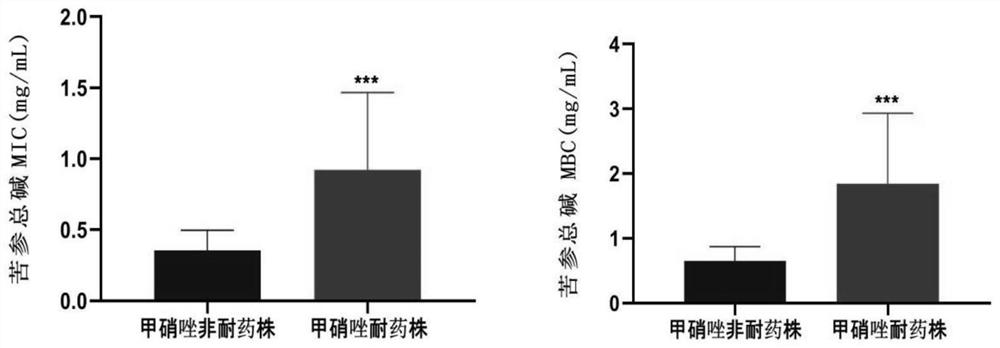
[0042] Example 2 Sophora flavescens preparation, metronidazole drug susceptibility test to Gardnerella clinical isolates
[0043] The MIC was determined by the microbroth dilution method in the CLSI (Clinical & Laboratory Standards Institute:) Guideline for Drug Susceptibility of Anaerobic Bacteria, and the standard strain of Bacteroides fragilis (ATCC 25285) was selected as the quality control bacteria.
[0044] (1) Preparation of antimicrobial drug dilution: thaw the pre-prepared antimicrobial drug stock solution or freshly prepared on the day of the test, and fill the test tube with quantitative sterilized distilled water or buffer solution as the blank solution of the dilution solution. Dilution preparation method: Use 1:2, 1:4 and 1:8 serial dilution methods to prepare the intermediate solution of antibacterial drugs (10×final concentration), and prepare the dilution according to this method to reduce dilution errors.
[0045]The final concentrations of the total alkalo...
Embodiment 3
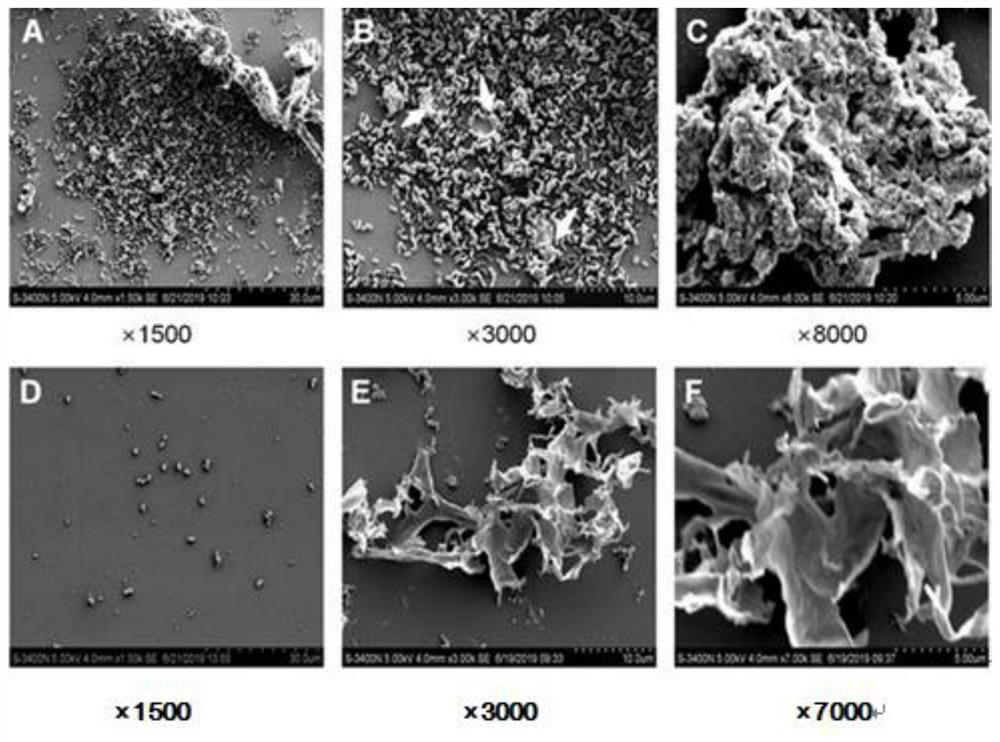
[0065] Example 3 Total alkaloids of Sophora flavescens inhibits Gardnerella biofilm
[0066] (1) Detection of minimum biofilm inhibitory concentration (MBIC) of total alkaloids of matrine on Gardnerella
[0067] 1) Bacterial liquid preparation: Take 10 μL of isolated and purified strains frozen in a -80°C refrigerator and inoculate them on casman solid medium, in 5% CO 2 , 37 ℃ static culture. After 48 hours of culture, pick a single colony and use a McFarland turbidimeter to prepare a bacterial solution of 0.5 McFarland units for use.
[0068] 2) Biofilm culture: 0.5 McFarland bacteria suspension (about 1.5×10 8 CFU / mL) was diluted 1:3 to reach 5×10 7 CFU / mL, add the diluted bacterial suspension to the prepared micro-dilution plate, add 10 μL to each well, so that the final concentration of bacteria is about 5×10 6 CFU / mL (in order to make the biofilm form better, increase the initial inoculum amount to 5 times that of the MIC experiment), put the inoculated micro-dilut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com