Indole-benzothiazole derivative as well as preparation method and application thereof
A technology for benzothiazole and derivatives, which is applied in the field of indole-benzothiazole derivatives and their preparation and application, can solve the problems of strong fluorescence response, small injection volume, and limited application of thiazole orange, and achieve high fluorescence intensity , strong specific effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
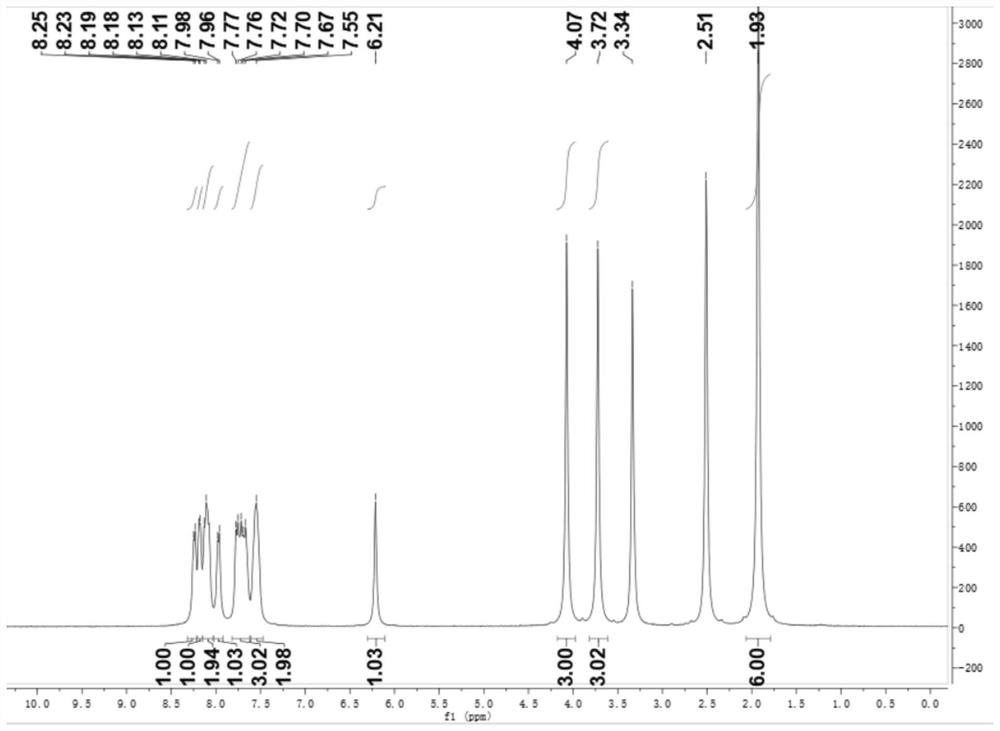
[0043] Embodiment 1: the synthesis of compound shown in formula (Ⅴ)
[0044] Weigh 1.0g (1.103mM) of 2-methylthiobenzothiazole (Ⅳ) and place it in an explosion-proof bottle, use 8mL of acetonitrile as a solvent, and ultrasonically vibrate to make the two evenly mixed; add twice the molar amount (2.206 mM) of methyl iodide, put the reaction system in an oil bath and turn on the magnetic stirring, the reaction temperature is 90°C, and the reaction time is 8h; after the reaction is completed, the reaction system is cooled to room temperature, and 10mL of ethyl acetate is added to fully shake , standing for 15min, the solid was precipitated, vacuum filtered, and the filter cake was rinsed with 5mL ethyl acetate, dried to obtain 1.07g white solid (Ⅴ), thin plate chromatography showed no by-products, and the crude yield was 60%. The reaction equation is as follows:
[0045]
Embodiment 2
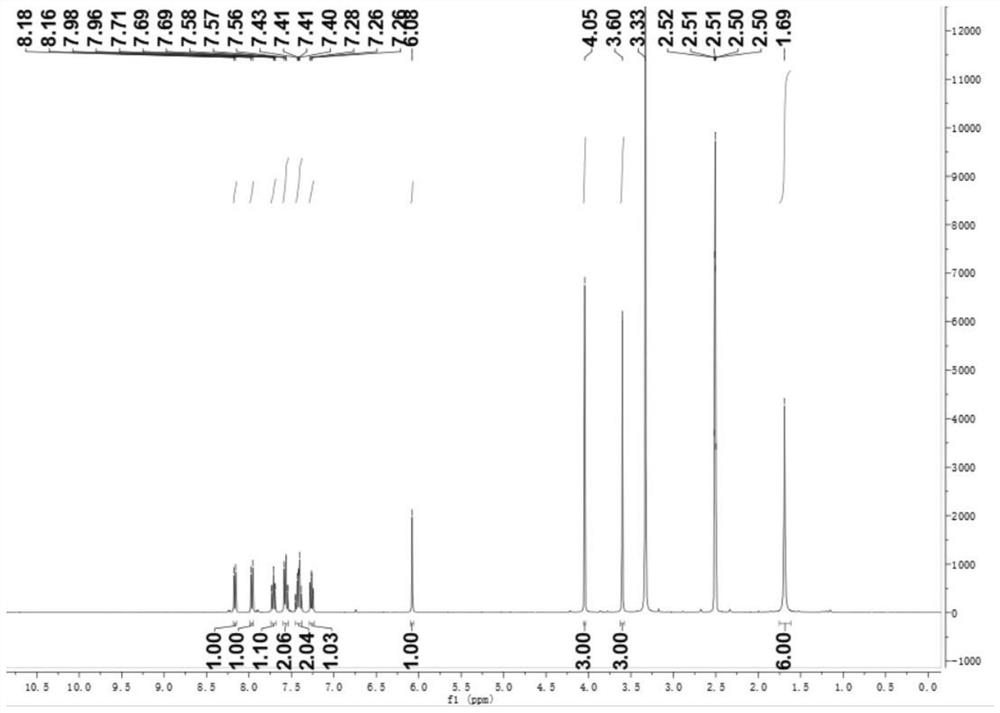
[0046] Embodiment 2: the synthesis of compound shown in formula (IX)
[0047] Weigh 0.2g (0.956mM) of 1,1,2-trimethyl-1H-benzo[e]indole (Ⅷ) into an explosion-proof bottle, use 2mL of acetonitrile as a solvent, and ultrasonicate for 3min to mix the two evenly. Add twice the molar amount (1.912mM) of methyl iodide under the condition of the hood, place the reaction system in a pot and turn on the magnetic stirring, the reaction temperature is 90°C, and the reaction time is 8h; after the reaction is completed, the reaction system is cooled After reaching room temperature, add 5 mL of ethyl acetate to shake fully, let stand for 15 min, precipitate solid, vacuum filter, and wash the filter cake with 3 mL of ethyl acetate, dry to obtain 0.29 g of light yellow solid (IX), thin plate chromatography shows no by-products , the crude yield was 86%. The reaction equation is as follows:
[0048]
Embodiment 3
[0049] Embodiment 3: the synthesis of compound shown in formula (XI)
[0050] Weigh 0.2g (1.240mM) of 2,3,3-trimethyl-3H-indole (Ⅹ) into an explosion-proof bottle, use 2mL of acetonitrile as a solvent, and ultrasonically mix the two for 3min. Add twice the molar amount (2.48mM) of methyl iodide, place the reaction system in a pot and turn on magnetic stirring, the reaction temperature is 90°C, and the reaction time is 8h; after the reaction is completed, the reaction system is cooled to room temperature, and then added 5mL of ethyl acetate was fully shaken, left to stand for 15min, the solid was precipitated, vacuum filtered, and the filter cake was rinsed with 3mL of ethyl acetate, dried to obtain 0.26g of pink solid (Ⅺ), thin plate chromatography showed no by-products, the crude yield was 70%. The reaction equation is as follows:
[0051]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com