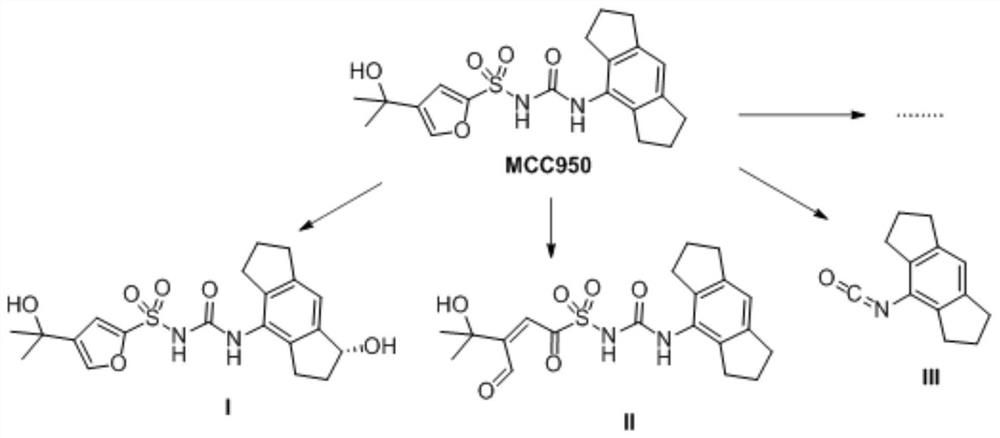
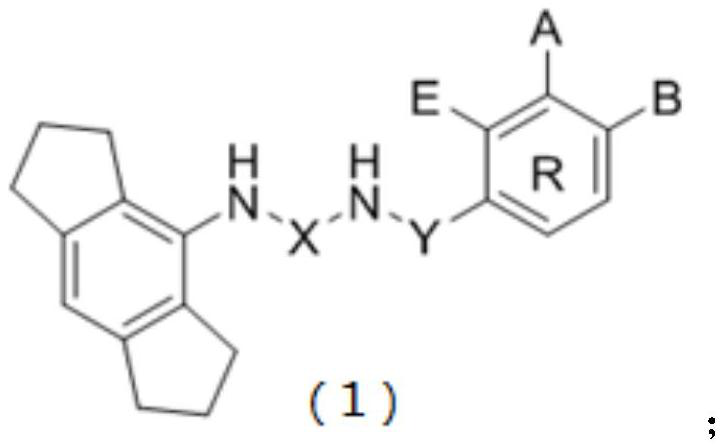
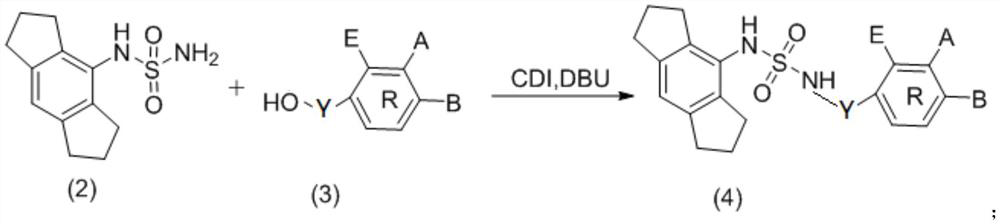
NLRP3 inflammasome inhibitor and preparation method and application thereof
A pharmacy and compound technology, applied in the field of NLRP3 inflammasome inhibitors and its preparation, can solve problems such as potential safety hazards, unclear off-target effects, and unclear elucidation of the specific mechanism of action of MCC950
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1 Preparation of (1,2,3,5,6,7-hexahydro-s-indan-4-yl) tert-butyl sulfamoylcarbamate (LW-01-7-1)
[0058] To a stirred solution of chlorosulfonyl isocyanate (CSI) (0.3ml, 2.65mmol) in anhydrous dichloromethane (3mL) was added tert-butanol (0.26, 2.65mmol) at 0°C, and after reacting for 30 minutes, the resulting solution was and triethylamine (0.375 mL, 2.65 mmol) were slowly added to 2 mL of a dichloromethane solution containing 1 equivalent of 1,2,3,5,6,7-hexahydro-s-indan-4-amine. The resulting reaction solution was warmed up to room temperature and reacted for 2 hours. The reaction mixture was diluted with 30 mL of dichloromethane, washed with 0.1N HCl and water. The organic layer was washed with Na 2 SO 4 Dry, filter, and concentrate in vacuo to give crude product. The residue was purified by silica gel column chromatography (PE / EA: 3 / 1).
Embodiment 2
[0059] Preparation of Example 2 LW-01-7-2
[0060] In order to remove the Boc protecting group, the above product (LW-01-7-1) was reacted in 6 mL of HCl:EA=1:2 (volume ratio) mixture at room temperature for 1 hour. It was then concentrated under vacuum. After neutralization with ammonia, the reaction mixture was diluted with 30 mL of dichloromethane and washed with water. The organic layer was washed with Na 2 SO 4 Dry, filter, and concentrate in vacuo to give crude product. The residue was purified by silica gel column chromatography (PE / EA: 2 / 1) to obtain the product (332 mg, 49.7%). The structural formula of the compound LW-01-7-2 is the formula (2) above.
Embodiment 3
[0061] Example 3 3-Bromo-N-(N-(1,2,3,5,6,7-hexahydro-s-indan-4-yl)sulfamoyl)benzamide (LW-01-18 -1)
[0062] Under nitrogen protection, 3-bromobenzoic acid (40mg, 0.2mmol) and CDI (65mg, 0.4mmol) were dissolved in THF (2mL), heated to reflux at 70°C for 45 minutes, cooled to room temperature and added LW-01-7 -2 (80 mg, 0.32 mmol) and DBU (0.09 mL, 0.6 mmol). The mixture was stirred at ambient temperature for 22 hours. The reaction mixture was diluted with 30 mL of dichloromethane, washed with 0.1N HCl and water. Na for organic layer 2 SO 4 Dry, filter, and concentrate in vacuo to give crude product. Purification by silica gel column chromatography (DCM / MeOH: 70 / 1) gave the product as a white solid (96 mg, 64%). 1 H NMR(400MHz,DMSO)δ12.03(s,1H),9.70(s,1H),8.04(s,1H),7.89(d,J=7.4Hz,1H),7.81(d,J=7.6Hz ,1H),7.48(t,J=7.6Hz,1H),7.00(s,1H),2.78(d,J=8.1Hz,8H),1.92–1.81(m,4H). 13 C NMR (151 MHz, DMSO) δ 164.85, 143.93, 141.05, 135.81, 135.12, 131.27, 131.14, 128.52, 127.64, 12...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com