Preparation method of perfluorinated nitrile compound
A compound, the technology of fluoronitrile, which is applied in the field of synthesis of perfluoronitrile compounds, can solve the problems of complicated steps, complicated operation and high production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
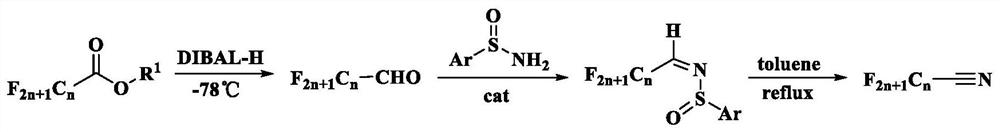
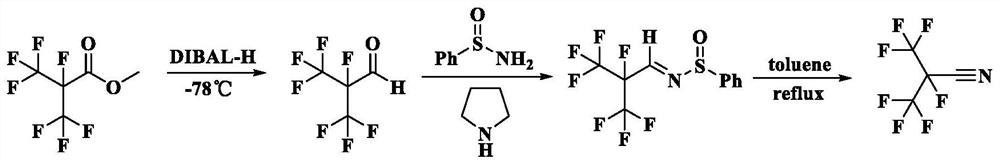
[0040] Please refer to figure 1 with figure 2 , the embodiment of this application will follow figure 1 The synthetic route shown prepares perfluoronitrile compound wherein, Ar is phenyl, R 1 For methyl, n is equal to 3. figure 2 Prepare the synthetic route figure of heptafluoroisobutyronitrile for the embodiment of the present application, the specific steps are as follows:
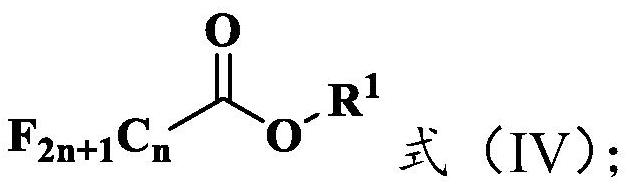
[0041] (1) At -78°C, add 1 molar equivalent of methyl heptafluoroisobutyrate (2.28g, 10mmol) and 25mL of the first solvent, anhydrous dichloromethane, into a round bottom flask, drop by drop 1.01mol / L reducing agent diisobutylaluminum hydride (DIBAL-H) cyclohexane solution (19.8mL, 20mmol), carry out the reduction reaction for 2h, then slowly add anhydrous methanol dropwise until no bubbles are generated, heat up to room temperature, and The reaction solution in the round bottom flask was added into 20mL dilute hydrochloric acid solution to quench the reaction, then extracted three times in batch...
Embodiment 2
[0047] According to the preparation method described in Example 1, the difference is only in the amount of reducing agent used in step (1) and the reduction reaction time. Table 1 shows the amount of reducing agent used, the reduction reaction time and the yield of heptafluoroisobutyraldehyde in the examples of the present application.
[0048] Table 1
[0049]
[0050] It can be seen from Table 1 that the amount of reducing agent diisobutylaluminum hydride is 1.2-3mol%, the reduction reaction time is 2-4h, and heptafluoroisobutylene can be prepared in excellent yield under the condition of -78°C. aldehyde.
Embodiment 3
[0052] According to the preparation method described in Example 1, the difference is only in the amount of phenylsulfinamide used in step (2), the selection and amount of catalyst, the temperature and time of the amination reaction. Table 2 shows the amount of phenylsulfinamide used in the examples of the application, the selection and amount of catalyst, the temperature and time of the amination reaction and the preparation of N-(2,3,3,3-tetrafluoro-2-(tri The yield of fluoromethyl) propylene) benzene sulfonamide.
[0053] Table 2
[0054]
[0055]
[0056] It can be seen from Table 2 that the addition equivalent of phenylsulfinamide is 1-1.5 molar equivalents, the addition equivalent of catalyst tetrahydropyrrole is 5-15 molar equivalents, the amination reaction time is 1-7h, and the reaction temperature is 40-70°C Both can produce 2-hexafluorobutyne N-(2,3,3,3-tetrafluoro-2-(trifluoromethyl)propylene)benzenesulfonamide.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com