African swine fever virus magnetic particle chemiluminiscence antibody detection kit and application thereof
An African swine fever virus and chemiluminescence technology, which is applied in the field of African swine fever virus magnetic particle chemiluminescence antibody detection kit, can solve the problems of low sensitivity, reducing the area of antigen or antibody interaction, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1 The formation of African swine fever virus magnetic particle chemiluminescent antibody detection kit
[0026] 1. Experimental method
[0027] 1.1 Preparation of ASFV p30 recombinant protein
[0028] 1.1.1 Propagation of strains for P30 recombinant protein production
[0029] Take the frozen preserved original strain E.coli-ASFV-p30 and inoculate it in LB liquid medium containing kanamycin (100 μg / mL), culture it at 37°C until the OD600nm value is 0.65, add 20% of the total volume after sampling Store in sterile glycerol aliquots.
[0030] 1.1.2 Prokaryotic expression of p30 recombinant protein
[0031] Preparation of bacterial solution: use 1 basic seed of genetically engineered bacteria E.coli-ASFV-p30, take an appropriate amount from the inoculation loop and streak on LB agar plate (containing 100 μg / mL kanamycin), culture at 37°C for 12 hours, as a primary seed. The primary seeds (single colony) were inoculated in 3 mL of LB medium (containing 100 μ...
Embodiment 2
[0108] Example 2 Detection and determination of African swine fever virus magnetic particle chemiluminescent antibody detection kit
[0109] 1. Test method
[0110] 1.1 Optimal reaction time selection
[0111] The reaction time of each step is 3 gradients of 5min, 10min and 15min. Detection by automatic chemiluminescence immunoassay analyzer. Determine the optimal reaction time based on the luminescence value.
[0112] 1.2 Determination of optimal sample volume
[0113] 1.2.1 Determination of the optimal sample volume of serum
[0114] For the detection of standard serum, the serum sample volume was set at 10 μL and 20 μL respectively, and detected with a fully automatic chemiluminescence immunoassay analyzer. According to the luminescence value and the detection range, determine the optimal amount of serum to add.
[0115] 1.2.2 Determination of the optimal amount of sample diluent
[0116] For the detection of standard serum, the amount of sample diluent was set to 10...
Embodiment 3
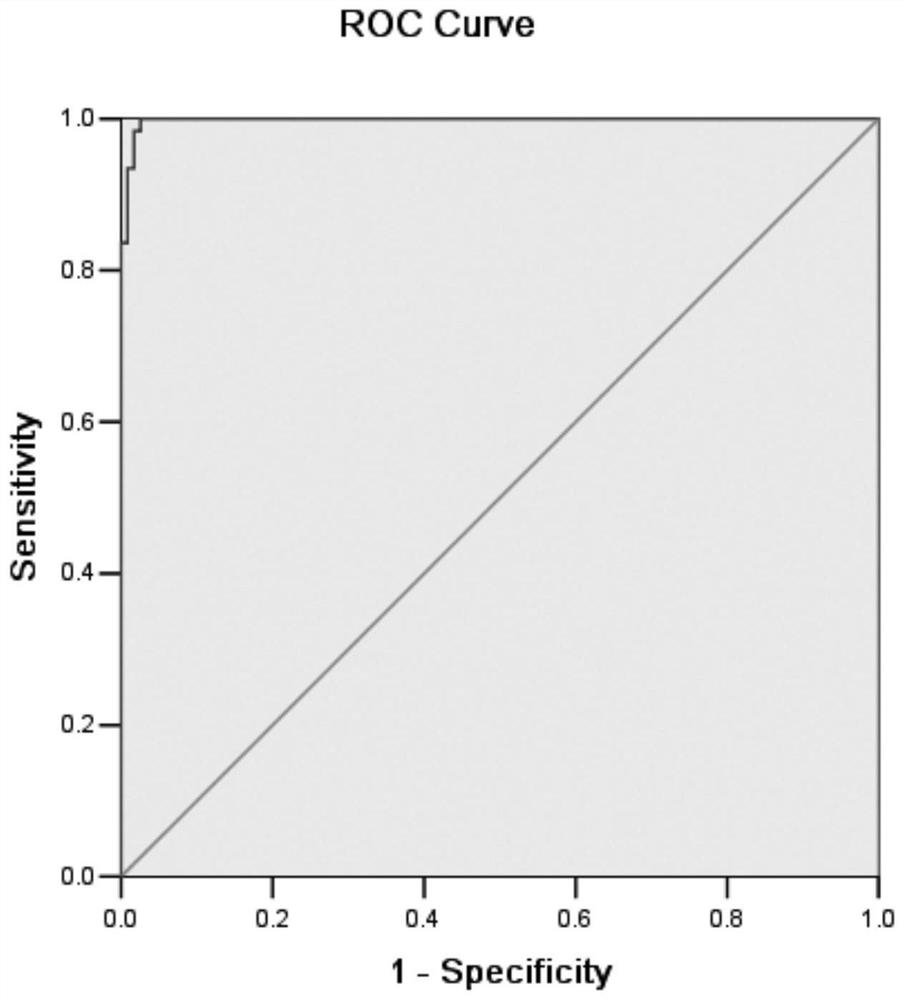
[0161] Example 3 Performance index of African swine fever virus magnetic particle chemiluminescent antibody detection kit
[0162] 1. Test method
[0163] 1.1 Specificity experiment
[0164] With 3 batches of ASFV antibody magnetic particle chemiluminescence kits prepared, to ASFV antibody positive serum (20 parts); ASFV antibody negative serum (10 parts); Seneca virus positive serum 5 parts; Classical swine fever virus positive serum 5 parts; 5 positive sera of reproductive and respiratory syndrome virus; 5 positive sera of foot-and-mouth disease virus type O; .
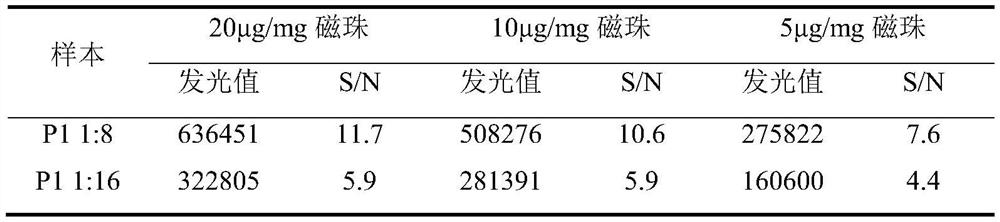
[0165] 1.2 Sensitivity test
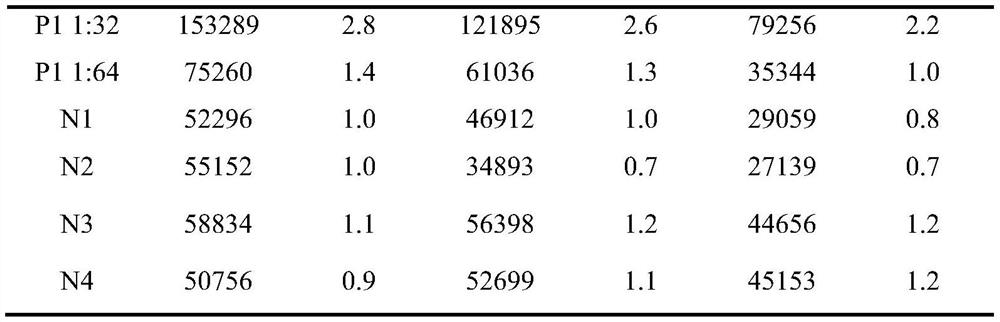
[0166] The positive standard serum was diluted with the sample diluent at a ratio of 1:2, 1:8, and 1:32, respectively, and named CP1, CP2, and CP3, respectively. As sensitive quality control serum, three batches of ASFV Ab magnetic particle chemistry were used. Luminescence kit detection. The S / CO of CP1 is required to be greater than 5.0, the S / CO of CP2 is between 1 and 2, and the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com